Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population
- PMID: 19918065
- PMCID: PMC2777968
- DOI: 10.1073/pnas.0911580106
Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population
Abstract
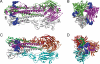
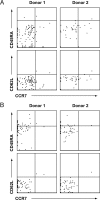
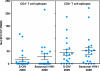
A major concern about the ongoing swine-origin H1N1 influenza virus (S-OIV) outbreak is that the virus may be so different from seasonal H1N1 that little immune protection exists in the human population. In this study, we examined the molecular basis for pre-existing immunity against S-OIV, namely the recognition of viral immune epitopes by T cells or B cells/antibodies that have been previously primed by circulating influenza strains. Using data from the Immune Epitope Database, we found that only 31% (8/26) of B-cell epitopes present in recently circulating H1N1 strains are conserved in the S-OIV, with only 17% (1/6) conserved in the hemagglutinin (HA) and neuraminidase (NA) surface proteins. In contrast, 69% (54/78) of the epitopes recognized by CD8(+) T cells are completely invariant. We further demonstrate experimentally that some memory T-cell immunity against S-OIV is present in the adult population and that such memory is of similar magnitude as the pre-existing memory against seasonal H1N1 influenza. Because protection from infection is antibody mediated, a new vaccine based on the specific S-OIV HA and NA proteins is likely to be required to prevent infection. However, T cells are known to blunt disease severity. Therefore, the conservation of a large fraction of T-cell epitopes suggests that the severity of an S-OIV infection, as far as it is determined by susceptibility of the virus to immune attack, would not differ much from that of seasonal flu. These results are consistent with reports about disease incidence, severity, and mortality rates associated with human S-OIV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus.J Virol. 2010 Apr;84(7):3312-9. doi: 10.1128/JVI.02226-09. Epub 2010 Jan 13. J Virol. 2010. PMID: 20071564 Free PMC article.
-
Immunoinformatic comparison of T-cell epitopes contained in novel swine-origin influenza A (H1N1) virus with epitopes in 2008-2009 conventional influenza vaccine.Vaccine. 2009 Sep 25;27(42):5740-7. doi: 10.1016/j.vaccine.2009.07.040. Epub 2009 Aug 4. Vaccine. 2009. PMID: 19660593
-
Highly conserved antigenic epitope regions of hemagglutinin and neuraminidase genes between 2009 H1N1 and seasonal H1N1 influenza: vaccine considerations.J Transl Med. 2013 Feb 22;11:47. doi: 10.1186/1479-5876-11-47. J Transl Med. 2013. PMID: 23433453 Free PMC article.
-
Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review.
-
CD4 T cells in protection from influenza virus: Viral antigen specificity and functional potential.Immunol Rev. 2018 Jul;284(1):91-105. doi: 10.1111/imr.12662. Immunol Rev. 2018. PMID: 29944766 Free PMC article. Review.
Cited by
-
Heterologous prime-boost H1N1 vaccination exacerbates disease following challenge with a mismatched H1N2 influenza virus in the swine model.Front Immunol. 2023 Oct 20;14:1253626. doi: 10.3389/fimmu.2023.1253626. eCollection 2023. Front Immunol. 2023. PMID: 37928521 Free PMC article.
-
Booster with Ad26.COV2.S or Omicron-adapted vaccine enhanced immunity and efficacy against SARS-CoV-2 Omicron in macaques.Nat Commun. 2023 Apr 7;14(1):1944. doi: 10.1038/s41467-023-37715-2. Nat Commun. 2023. PMID: 37029141 Free PMC article.
-
SARS-CoV-2-The Role of Natural Immunity: A Narrative Review.J Clin Med. 2022 Oct 25;11(21):6272. doi: 10.3390/jcm11216272. J Clin Med. 2022. PMID: 36362500 Free PMC article. Review.
-
FLU-v, a Broad-Spectrum Influenza Vaccine, Induces Cross-Reactive Cellular Immune Responses in Humans Measured by Dual IFN-γ and Granzyme B ELISpot Assay.Vaccines (Basel). 2022 Sep 14;10(9):1528. doi: 10.3390/vaccines10091528. Vaccines (Basel). 2022. PMID: 36146606 Free PMC article.
-
The role of cell-mediated immunity against influenza and its implications for vaccine evaluation.Front Immunol. 2022 Aug 16;13:959379. doi: 10.3389/fimmu.2022.959379. eCollection 2022. Front Immunol. 2022. PMID: 36052083 Free PMC article. Review.
References
-
- Cohen J, Enserink M. Swine flu. After delays, WHO agrees: The 2009 pandemic has begun. Science. 2009;324:1496–1497. - PubMed
-
- Centers for Disease Control and Prevention. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR. 2009;58:521–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

