Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP
- PMID: 19911184
- PMCID: PMC2808512
- DOI: 10.1007/s00401-009-0609-x
Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP
Abstract
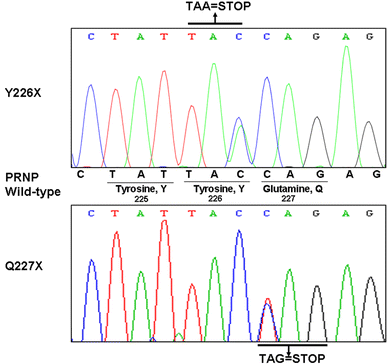
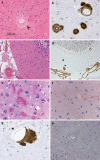
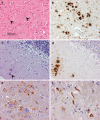
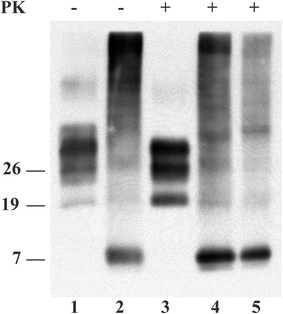
Stop codon mutations in the gene encoding the prion protein (PRNP) are very rare and have thus far only been described in two patients with prion protein cerebral amyloid angiopathy (PrP-CAA). In this report, we describe the clinical, histopathological and pathological prion protein (PrP(Sc)) characteristics of two Dutch patients carrying novel adjacent stop codon mutations in the C-terminal part of PRNP, resulting in either case in hereditary prion protein amyloidoses, but with strikingly different clinicopathological phenotypes. The patient with the shortest disease duration (27 months) carried a Y226X mutation and showed PrP-CAA without any neurofibrillary lesions, whereas the patient with the longest disease duration (72 months) had a Q227X mutation and showed an unusual Gerstmann-Sträussler-Scheinker disease phenotype with numerous cerebral multicentric amyloid plaques and severe neurofibrillary lesions without PrP-CAA. Western blot analysis in the patient with the Q227X mutation demonstrated the presence of a 7 kDa unglycosylated PrP(Sc) fragment truncated at both the N- and C-terminal ends. Our observations expand the spectrum of clinicopathological phenotypes associated with PRNP mutations and show that a single tyrosine residue difference in the PrP C-terminus may significantly affect the site of amyloid deposition and the overall phenotypic expression of the prion disease. Furthermore, it confirms that the absence of the glycosylphosphatidylinositol anchor in PrP predisposes to amyloid plaque formation.
Figures
Similar articles
-
Hereditary prion protein amyloidoses.Clin Lab Med. 2003 Mar;23(1):65-85, viii. doi: 10.1016/s0272-2712(02)00064-1. Clin Lab Med. 2003. PMID: 12733425 Review.
-
A novel seven-octapeptide repeat insertion in the prion protein gene (PRNP) in a Dutch pedigree with Gerstmann-Sträussler-Scheinker disease phenotype: comparison with similar cases from the literature.Acta Neuropathol. 2011 Jan;121(1):59-68. doi: 10.1007/s00401-010-0656-3. Epub 2010 Mar 3. Acta Neuropathol. 2011. PMID: 20198483 Free PMC article. Review.
-
Characterization of Anchorless Human PrP With Q227X Stop Mutation Linked to Gerstmann-Sträussler-Scheinker Syndrome In Vivo and In Vitro.Mol Neurobiol. 2021 Jan;58(1):21-33. doi: 10.1007/s12035-020-02098-8. Epub 2020 Sep 5. Mol Neurobiol. 2021. PMID: 32889654 Free PMC article.
-
A novel PRNP-P105S mutation associated with atypical prion disease and a rare PrPSc conformation.Neurology. 2008 Oct 28;71(18):1431-8. doi: 10.1212/01.wnl.0000330237.94742.fa. Neurology. 2008. PMID: 18955686 Free PMC article.
-
Prion protein amyloidosis.Brain Pathol. 1996 Apr;6(2):127-45. doi: 10.1111/j.1750-3639.1996.tb00796.x. Brain Pathol. 1996. PMID: 8737929 Review.
Cited by
-
An overview of human prion diseases.Virol J. 2011 Dec 24;8:559. doi: 10.1186/1743-422X-8-559. Virol J. 2011. PMID: 22196171 Free PMC article. Review.
-
Proteolytic processing of the prion protein in health and disease.Am J Neurodegener Dis. 2012;1(1):15-31. Epub 2012 May 15. Am J Neurodegener Dis. 2012. PMID: 23383379 Free PMC article.
-
Hereditary Human Prion Diseases: an Update.Mol Neurobiol. 2017 Aug;54(6):4138-4149. doi: 10.1007/s12035-016-9918-y. Epub 2016 Jun 20. Mol Neurobiol. 2017. PMID: 27324792 Review.
-
Clinical and neuropathological phenotype associated with the novel V189I mutation in the prion protein gene.Acta Neuropathol Commun. 2019 Jan 3;7(1):1. doi: 10.1186/s40478-018-0656-4. Acta Neuropathol Commun. 2019. PMID: 30606247 Free PMC article.
-
Fatal transmissible amyloid encephalopathy: a new type of prion disease associated with lack of prion protein membrane anchoring.PLoS Pathog. 2010 Mar 5;6(3):e1000800. doi: 10.1371/journal.ppat.1000800. PLoS Pathog. 2010. PMID: 20221436 Free PMC article.
References
-
- Capellari S, Cardone F, Notari S, et al. Creutzfeldt-Jakob disease associated with the R208H mutation in the prion protein gene. Neurology. 2005;64:905–907. - PubMed
-
- Capellari S, Vital C, Parchi P, et al. Familial prion disease with a novel 144-bp insertion in the prion protein gene in a Basque family. Neurology. 1997;49:133–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

