Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression
- PMID: 19907077
- PMCID: PMC2786793
- DOI: 10.1172/JCI39335
Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression
Abstract
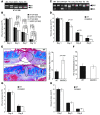
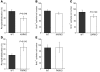
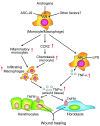
Cutaneous wounds heal more slowly in elderly males than in elderly females, suggesting a role for sex hormones in the healing process. Indeed, androgen/androgen receptor (AR) signaling has been shown to inhibit cutaneous wound healing. AR is expressed in several cell types in healing skin, including keratinocytes, dermal fibroblasts, and infiltrating macrophages, but the exact role of androgen/AR signaling in these different cell types remains unclear. To address this question, we generated and studied cutaneous wound healing in cell-specific AR knockout (ARKO) mice. General and myeloid-specific ARKO mice exhibited accelerated wound healing compared with WT mice, whereas keratinocyte- and fibroblast-specific ARKO mice did not. Importantly, the rate of wound healing in the general ARKO mice was dependent on AR and not serum androgen levels. Interestingly, although dispensable for wound closure, keratinocyte AR promoted re-epithelialization, while fibroblast AR suppressed it. Further analysis indicated that AR suppressed wound healing by enhancing the inflammatory response through a localized increase in TNF-alpha expression. Furthermore, AR enhanced local TNF-alpha expression via multiple mechanisms, including increasing the inflammatory monocyte population, enhancing monocyte chemotaxis by upregulating CCR2 expression, and enhancing TNF-alpha expression in macrophages. Finally, targeting AR by topical application of a compound (ASC-J9) that degrades AR protein resulted in accelerated healing, suggesting a potential new therapeutic approach that may lead to better treatment of wound healing.
Figures



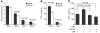
Similar articles
-
New therapy via targeting androgen receptor in monocytes/macrophages to battle atherosclerosis.Hypertension. 2014 Jun;63(6):1345-53. doi: 10.1161/HYPERTENSIONAHA.113.02804. Epub 2014 Mar 31. Hypertension. 2014. PMID: 24688120 Free PMC article.
-
Androgen actions in mouse wound healing: Minimal in vivo effects of local antiandrogen delivery.Wound Repair Regen. 2016 May;24(3):478-88. doi: 10.1111/wrr.12420. Epub 2016 May 6. Wound Repair Regen. 2016. PMID: 26873751
-
Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking β-catenin nuclear translocation and cross-talk with TGF-β signaling in keratinocytes.Wound Repair Regen. 2012 Jan-Feb;20(1):61-73. doi: 10.1111/j.1524-475X.2011.00757.x. Wound Repair Regen. 2012. PMID: 22276587 Free PMC article.
-
Androgen receptor (AR) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: lessons learned from mice lacking AR in specific cells.Nucl Recept Signal. 2013 Aug 19;11:e001. doi: 10.1621/nrs.11001. eCollection 2013. Nucl Recept Signal. 2013. PMID: 24653668 Free PMC article. Review.
-
A review of the effects and molecular mechanisms of dimethylcurcumin (ASC-J9) on androgen receptor-related diseases.Chem Biol Drug Des. 2021 Apr;97(4):821-835. doi: 10.1111/cbdd.13811. Epub 2021 Jan 11. Chem Biol Drug Des. 2021. PMID: 33277796 Review.
Cited by
-
Shaping the immune landscape: Multidimensional environmental stimuli refine macrophage polarization and foster revolutionary approaches in tissue regeneration.Heliyon. 2024 Aug 30;10(17):e37192. doi: 10.1016/j.heliyon.2024.e37192. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296009 Free PMC article. Review.
-
Nuclear Receptors and Stress Response Pathways Associated with the Development of Oral Mucositis Induced by Antineoplastic Agents.Pharmaceuticals (Basel). 2024 Aug 20;17(8):1086. doi: 10.3390/ph17081086. Pharmaceuticals (Basel). 2024. PMID: 39204191 Free PMC article.
-
Macrophages of multiple hematopoietic origins reside in the developing prostate.Development. 2024 Aug 15;151(16):dev203070. doi: 10.1242/dev.203070. Epub 2024 Aug 29. Development. 2024. PMID: 39082371 Free PMC article.
-
Hallmarks of sex bias in immuno-oncology: mechanisms and therapeutic implications.Nat Rev Cancer. 2024 May;24(5):338-355. doi: 10.1038/s41568-024-00680-z. Epub 2024 Apr 8. Nat Rev Cancer. 2024. PMID: 38589557
-
RISING STARS: Androgens and immune cell function.J Endocrinol. 2024 Apr 29;261(3):e230398. doi: 10.1530/JOE-23-0398. Print 2024 Jun 1. J Endocrinol. 2024. PMID: 38579776 Free PMC article. Review.
References
-
- Taylor R.J., Taylor A.D., Smyth J.V. Using an artificial neural network to predict healing times and risk factors for venous leg ulcers. J. Wound Care. 2002;11:101–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

