Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection
- PMID: 19906920
- PMCID: PMC2812346
- DOI: 10.1128/JVI.01281-09
Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection
Abstract
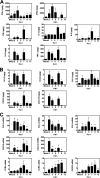
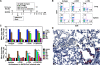
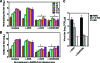
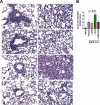
We characterized the cellular immune response to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in 12- to 14-month-old BALB/c mice, a model that mimics features of the human disease. Following intranasal administration, the virus replicated in the lungs, with peak titers on day 2 postinfection. Enhanced production of cytokines (tumor necrosis factor alpha [TNF-alpha] and interleukin-6 [IL-6]) and chemokines (CXCL10, CCL2, CCL3, and CCL5) correlated with migration of NK cells, macrophages, and plasmacytoid dendritic cells (pDC) into the lungs. By day 7, histopathologic evidence of pneumonitis was seen in the lungs when viral clearance occurred. At this time, a second wave of enhanced production of cytokines (TNF-alpha, IL-6, gamma interferon [IFN-gamma], IL-2, and IL-5), chemokines (CXCL9, CXCL10, CCL2, CCL3, and CCL5), and receptors (CXCR3, CCR2, and CCR5), was detected in the lungs, associated with an influx of T lymphocytes. Depletion of CD8(+) T cells at the time of infection did not affect viral replication or clearance. However, depletion of CD4(+) T cells resulted in an enhanced immune-mediated interstitial pneumonitis and delayed clearance of SARS-CoV from the lungs, which was associated with reduced neutralizing antibody and cytokine production and reduced pulmonary recruitment of lymphocytes. Innate defense mechanisms are able to control SARS-CoV infection in the absence of CD4(+) and CD8(+) T cells and antibodies. Our findings provide new insights into the pathogenesis of SARS, demonstrating the important role of CD4(+) but not CD8(+) T cells in primary SARS-CoV infection in this model.
Figures


Similar articles
-
Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice.J Immunol. 2004 Sep 15;173(6):4030-9. doi: 10.4049/jimmunol.173.6.4030. J Immunol. 2004. PMID: 15356152
-
Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection.J Virol. 2014 Oct;88(19):11034-44. doi: 10.1128/JVI.01505-14. Epub 2014 Jul 23. J Virol. 2014. PMID: 25056892 Free PMC article.
-
Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice.Am J Pathol. 2008 Jun;172(6):1625-37. doi: 10.2353/ajpath.2008.071060. Epub 2008 May 8. Am J Pathol. 2008. PMID: 18467696 Free PMC article.
-
Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: Case series of four critically ill patients treated with leronlimab.J Transl Autoimmun. 2021;4:100083. doi: 10.1016/j.jtauto.2021.100083. Epub 2021 Jan 6. J Transl Autoimmun. 2021. PMID: 33521616 Free PMC article. Review.
-
T cell-mediated immune response to respiratory coronaviruses.Immunol Res. 2014 Aug;59(1-3):118-28. doi: 10.1007/s12026-014-8534-z. Immunol Res. 2014. PMID: 24845462 Free PMC article. Review.
Cited by
-
Cross-immunity between respiratory coronaviruses may limit COVID-19 fatalities.Med Hypotheses. 2020 Nov;144:110049. doi: 10.1016/j.mehy.2020.110049. Epub 2020 Jun 30. Med Hypotheses. 2020. PMID: 32758887 Free PMC article.
-
SARS coronavirus 3b accessory protein modulates transcriptional activity of RUNX1b.PLoS One. 2012;7(1):e29542. doi: 10.1371/journal.pone.0029542. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22253733 Free PMC article.
-
Expanding roles for CD4⁺ T cells in immunity to viruses.Nat Rev Immunol. 2012 Jan 20;12(2):136-48. doi: 10.1038/nri3152. Nat Rev Immunol. 2012. PMID: 22266691 Free PMC article. Review.
-
Investigation of immune cells on elimination of pulmonary-Infected COVID-19 and important role of innate immunity, phagocytes.Rev Med Virol. 2021 Mar;31(2):e2158. doi: 10.1002/rmv.2158. Epub 2020 Sep 18. Rev Med Virol. 2021. PMID: 32946131 Free PMC article. Review.
-
Reduced Expression of Autophagy Markers and Expansion of Myeloid-Derived Suppressor Cells Correlate With Poor T Cell Response in Severe COVID-19 Patients.Front Immunol. 2021 Feb 22;12:614599. doi: 10.3389/fimmu.2021.614599. eCollection 2021. Front Immunol. 2021. PMID: 33692788 Free PMC article.
References
-
- Brown, D. M., E. Roman, and S. L. Swain. 2004. CD4 T cell responses to influenza infection. Semin. Immunol. 16:171-177. - PubMed
-
- Cameron, M. J., L. Ran, L. Xu, A. Danesh, J. F. Bermejo-Martin, C. M. Cameron, M. P. Muller, W. L. Gold, S. E. Richardson, S. M. Poutanen, B. M. Willey, M. E. DeVries, Y. Fang, C. Seneviratne, S. E. Bosinger, D. Persad, P. Wilkinson, L. D. Greller, R. Somogyi, A. Humar, S. Keshavjee, M. Louie, M. B. Loeb, J. Brunton, A. J. McGeer, and D. J. Kelvin. 2007. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 81:8692-8706. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

