Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development
- PMID: 19906862
- PMCID: PMC2778742
- DOI: 10.1242/dev.042291
Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development
Abstract
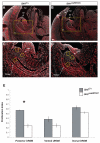
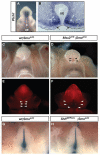
Malformations of the external genitalia are among the most common congenital anomalies in humans. The urogenital and anorectal sinuses develop from the embryonic cloaca, and the penis and clitoris develop from the genital tubercle. Within the genital tubercle, the endodermally derived urethral epithelium functions as an organizer and expresses sonic hedgehog (Shh). Shh knockout mice lack external genitalia and have a persistent cloaca. This identified an early requirement for Shh, but precluded analysis of its later role in the genital tubercle. We conducted temporally controlled deletions of Shh and report that Shh is required continuously through the onset of sexual differentiation. Shh function is divisible into two temporal phases; an anogenital phase, during which Shh regulates outgrowth and patterning of the genital tubercle and septation of the cloaca, and a later external genital phase, during which Shh regulates urethral tube closure. Disruption of Shh function during the anogenital phase causes coordinated anorectal and genitourinary malformations, whereas inactivation during the external genital phase causes hypospadias. Shh directs cloacal septation by promoting cell proliferation in adjacent urorectal septum mesenchyme. Additionally, conditional inactivation of smoothened in the genital ectoderm and cloacal/urethral endoderm shows that the ectoderm is a direct target of Shh and is required for urethral tube closure, highlighting a novel role for genital ectoderm in urethragenesis. Identification of the stages during which disruption of Shh results in either isolated or coordinated malformations of anorectal and external genital organs provides a new tool for investigating the etiology of anogenital malformations in humans.
Figures




Similar articles
-
Development of the external genitalia: conserved and divergent mechanisms of appendage patterning.Dev Dyn. 2011 May;240(5):1108-15. doi: 10.1002/dvdy.22631. Epub 2011 Apr 4. Dev Dyn. 2011. PMID: 21465625 Free PMC article. Review.
-
Sonic hedgehog signaling from the urethral epithelium controls external genital development.Dev Biol. 2002 Jul 1;247(1):26-46. doi: 10.1006/dbio.2002.0668. Dev Biol. 2002. PMID: 12074550
-
Functional and phylogenetic analysis shows that Fgf8 is a marker of genital induction in mammals but is not required for external genital development.Development. 2009 Aug;136(15):2643-51. doi: 10.1242/dev.036830. Development. 2009. PMID: 19592577 Free PMC article.
-
Foxa1 and Foxa2 orchestrate development of the urethral tube and division of the embryonic cloaca through an autoregulatory loop with Shh.Dev Biol. 2020 Sep 1;465(1):23-30. doi: 10.1016/j.ydbio.2020.06.009. Epub 2020 Jul 6. Dev Biol. 2020. PMID: 32645357 Free PMC article.
-
Molecular genetic cascades for external genitalia formation: an emerging organogenesis program.Dev Dyn. 2006 Jul;235(7):1738-52. doi: 10.1002/dvdy.20807. Dev Dyn. 2006. PMID: 16598715 Review.
Cited by
-
Bmp7 functions via a polarity mechanism to promote cloacal septation.PLoS One. 2012;7(1):e29372. doi: 10.1371/journal.pone.0029372. Epub 2012 Jan 13. PLoS One. 2012. PMID: 22253716 Free PMC article.
-
Development of the external genitalia: conserved and divergent mechanisms of appendage patterning.Dev Dyn. 2011 May;240(5):1108-15. doi: 10.1002/dvdy.22631. Epub 2011 Apr 4. Dev Dyn. 2011. PMID: 21465625 Free PMC article. Review.
-
An extra-genital cell population contributes to urethra closure during mouse penis development.bioRxiv [Preprint]. 2023 Nov 11:2023.11.09.564741. doi: 10.1101/2023.11.09.564741. bioRxiv. 2023. Update in: Sci Adv. 2024 Dec 6;10(49):eadp0673. doi: 10.1126/sciadv.adp0673 PMID: 37986842 Free PMC article. Updated. Preprint.
-
Tissue-specific roles of Fgfr2 in development of the external genitalia.Development. 2015 Jun 15;142(12):2203-12. doi: 10.1242/dev.119891. Development. 2015. PMID: 26081573 Free PMC article.
-
Development and functional characterization of a lncRNA-HIT conditional loss of function allele.Genesis. 2020 Mar;58(3-4):e23351. doi: 10.1002/dvg.23351. Epub 2019 Dec 14. Genesis. 2020. PMID: 31838787 Free PMC article.
References
-
- Arsic D., Qi B. Q., Beasley S. W. (2002). Hedgehog in the human: a possible explanation for the VATER association. J. Paediatr. Child Health 38, 117-121 - PubMed
-
- Cassini P., Montironi A., Botti S., Hori T., Okhawa H., Stella A., Andersson L., Giuffra E. (2005). Genetic analysis of anal atresia in pigs: evidence for segregation at two main loci. Mamm. Genome 16, 164-170 - PubMed
-
- Cheng W., Yeung C. K., Ng Y. K., Zhang J. R., Hui C. C., Kim P. C. (2008). Sonic Hedgehog mediator Gli2 regulates bladder mesenchymal patterning. J. Urol. 180, 1543-1450 - PubMed
-
- Dassule H. R., Lewis P., Bei M., Maas R., McMahon A. P. (2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775-4785 - PubMed
-
- Dawrant M. J., Giles S., Bannigan J., Puri P. (2008). Adriamycin produces a reproducible teratogenic model of gastrointestinal atresia in the mouse. Pediatr. Surg. Int. 24, 731-735 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

