The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli
- PMID: 19897651
- PMCID: PMC2805327
- DOI: 10.1128/JB.01144-09
The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli
Abstract
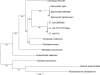
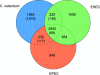
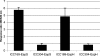
Citrobacter rodentium (formally Citrobacter freundii biotype 4280) is a highly infectious pathogen that causes colitis and transmissible colonic hyperplasia in mice. In common with enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC, respectively), C. rodentium exploits a type III secretion system (T3SS) to induce attaching and effacing (A/E) lesions that are essential for virulence. Here, we report the fully annotated genome sequence of the 5.3-Mb chromosome and four plasmids harbored by C. rodentium strain ICC168. The genome sequence revealed key information about the phylogeny of C. rodentium and identified 1,585 C. rodentium-specific (without orthologues in EPEC or EHEC) coding sequences, 10 prophage-like regions, and 17 genomic islands, including the locus for enterocyte effacement (LEE) region, which encodes a T3SS and effector proteins. Among the 29 T3SS effectors found in C. rodentium are all 22 of the core effectors of EPEC strain E2348/69. In addition, we identified a novel C. rodentium effector, named EspS. C. rodentium harbors two type VI secretion systems (T6SS) (CTS1 and CTS2), while EHEC contains only one T6SS (EHS). Our analysis suggests that C. rodentium and EPEC/EHEC have converged on a common host infection strategy through access to a common pool of mobile DNA and that C. rodentium has lost gene functions associated with a previous pathogenic niche.
Figures



Similar articles
-
Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens.Infect Immun. 2001 Oct;69(10):6323-35. doi: 10.1128/IAI.69.10.6323-6335.2001. Infect Immun. 2001. PMID: 11553577 Free PMC article.
-
The Prophages of Citrobacter rodentium Represent a Conserved Family of Horizontally Acquired Mobile Genetic Elements Associated with Enteric Evolution towards Pathogenicity.J Bacteriol. 2019 Apr 9;201(9):e00638-18. doi: 10.1128/JB.00638-18. Print 2019 May 1. J Bacteriol. 2019. PMID: 30782635 Free PMC article.
-
Genomic analysis of the PAI ICL3 locus in pathogenic LEE-negative Shiga toxin-producing Escherichia coli and Citrobacter rodentium.Microbiology (Reading). 2009 Apr;155(Pt 4):1016-1027. doi: 10.1099/mic.0.026807-0. Microbiology (Reading). 2009. PMID: 19332804
-
Type Three Secretion System in Attaching and Effacing Pathogens.Front Cell Infect Microbiol. 2016 Oct 21;6:129. doi: 10.3389/fcimb.2016.00129. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27818950 Free PMC article. Review.
-
[Plasticity of bacterial genomes: pathogenicity islands and the locus of enterocyte effacement (LEE)].Berl Munch Tierarztl Wochenschr. 2004 Mar-Apr;117(3-4):116-29. Berl Munch Tierarztl Wochenschr. 2004. PMID: 15046458 Review. German.
Cited by
-
The enteropathogenic E. coli (EPEC) Tir effector inhibits NF-κB activity by targeting TNFα receptor-associated factors.PLoS Pathog. 2011 Dec;7(12):e1002414. doi: 10.1371/journal.ppat.1002414. Epub 2011 Dec 1. PLoS Pathog. 2011. PMID: 22144899 Free PMC article.
-
A distinct regulatory sequence is essential for the expression of a subset of nle genes in attaching and effacing Escherichia coli.J Bacteriol. 2012 Oct;194(20):5589-603. doi: 10.1128/JB.00190-12. Epub 2012 Aug 17. J Bacteriol. 2012. PMID: 22904277 Free PMC article.
-
Complete Sequences of mcr-1-Harboring Plasmids from Extended-Spectrum-β-Lactamase- and Carbapenemase-Producing Enterobacteriaceae.Antimicrob Agents Chemother. 2016 Jun 20;60(7):4351-4. doi: 10.1128/AAC.00550-16. Print 2016 Jul. Antimicrob Agents Chemother. 2016. PMID: 27090180 Free PMC article.
-
Citrobacter rodentium Infection Induces Persistent Molecular Changes and Interferon Gamma-Dependent Major Histocompatibility Complex Class II Expression in the Colonic Epithelium.mBio. 2021 Feb 22;13(1):e0323321. doi: 10.1128/mbio.03233-21. Epub 2022 Feb 1. mBio. 2021. PMID: 35100877 Free PMC article.
-
Association between intestinal microbiota and inflammatory bowel disease.Animal Model Exp Med. 2022 Dec;5(4):311-322. doi: 10.1002/ame2.12255. Epub 2022 Jul 8. Animal Model Exp Med. 2022. PMID: 35808814 Free PMC article. Review.
References
-
- Barthold, S. W., G. L. Coleman, P. N. Bhatt, G. W. Osbaldiston, and A. M. Jonas. 1976. The etiology of transmissible murine colonic hyperplasia. Lab Anim. Sci. 26:889-894. - PubMed
-
- Barthold, S. W., G. W. Osbaldiston, and A. M. Jonas. 1977. Dietary, bacterial, and host genetic interactions in the pathogenesis of transmissible murine colonic hyperplasia. Lab Anim. Sci. 27:938-945. - PubMed
MeSH terms
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

