Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis
- PMID: 19897576
- PMCID: PMC5070714
- DOI: 10.1182/blood-2009-04-218735
Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis
Erratum in
- Blood. 2010 Sep 23;116(12):2195
Abstract
Tissue infiltration of phagocytes exacerbates several human pathologies including chronic inflammations or cancers. However, the mechanisms involved in macrophage migration through interstitial tissues are poorly understood. We investigated the role of Hck, a Src-family kinase involved in the organization of matrix adhesion and degradation structures called podosomes. In Hck(-/-) mice submitted to peritonitis, we found that macrophages accumulated in interstitial tissues and barely reached the peritoneal cavity. In vitro, 3-dimensional (3D) migration and matrix degradation abilities, 2 protease-dependent properties of bone marrow-derived macrophages (BMDMs), were affected in Hck(-/-) BMDMs. These macrophages formed few and undersized podosome rosettes and, consequently, had reduced matrix proteolysis operating underneath despite normal expression and activity of matrix metalloproteases. Finally, in fibroblasts unable to infiltrate matrix, ectopic expression of Hck provided the gain-of-3D migration function, which correlated positively with formation of podosome rosettes. In conclusion, spatial organization of podosomes as large rosettes, proteolytic degradation of extracellular matrix, and 3D migration appeared to be functionally linked and regulated by Hck in macrophages. Hck, as the first protein combining a phagocyte-limited expression with a role in 3D migration, could be a target for new anti-inflammatory and antitumor molecules.
Figures
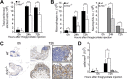
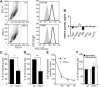
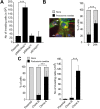
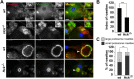
Similar articles
-
Macrophage podosomes go 3D.Eur J Cell Biol. 2011 Feb-Mar;90(2-3):224-36. doi: 10.1016/j.ejcb.2010.07.011. Eur J Cell Biol. 2011. PMID: 20801545
-
Hck contributes to bone homeostasis by controlling the recruitment of osteoclast precursors.FASEB J. 2013 Sep;27(9):3608-18. doi: 10.1096/fj.13-232736. Epub 2013 Jun 6. FASEB J. 2013. PMID: 23742809 Free PMC article.
-
Re-arrangements of podosome structures are observed when Hck is activated in myeloid cells.Eur J Cell Biol. 2006 Apr;85(3-4):327-32. doi: 10.1016/j.ejcb.2005.09.012. Epub 2005 Oct 19. Eur J Cell Biol. 2006. PMID: 16546576
-
Hematopoietic cell kinase (Hck) isoforms and phagocyte duties - from signaling and actin reorganization to migration and phagocytosis.Eur J Cell Biol. 2008 Sep;87(8-9):527-42. doi: 10.1016/j.ejcb.2008.03.008. Epub 2008 Jun 5. Eur J Cell Biol. 2008. PMID: 18538446 Review.
-
Control of macrophage 3D migration: a therapeutic challenge to limit tissue infiltration.Immunol Rev. 2014 Nov;262(1):216-31. doi: 10.1111/imr.12214. Immunol Rev. 2014. PMID: 25319337 Review.
Cited by
-
Recombinant protein rVP1 upregulates BECN1-independent autophagy, MAPK1/3 phosphorylation and MMP9 activity via WIPI1/WIPI2 to promote macrophage migration.Autophagy. 2013 Jan;9(1):5-19. doi: 10.4161/auto.22379. Epub 2012 Oct 10. Autophagy. 2013. PMID: 23051912 Free PMC article.
-
WASP and WAVE family proteins: friends or foes in cancer invasion?Cancer Sci. 2010 Oct;101(10):2093-104. doi: 10.1111/j.1349-7006.2010.01654.x. Epub 2010 Aug 12. Cancer Sci. 2010. PMID: 20707804 Free PMC article. Review.
-
Nanomechanical measurement of adhesion and migration of leukemia cells with phorbol 12-myristate 13-acetate treatment.Int J Nanomedicine. 2016 Dec 7;11:6533-6545. doi: 10.2147/IJN.S118065. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27994457 Free PMC article.
-
Frustrated phagocytosis on micro-patterned immune complexes to characterize lysosome movements in live macrophages.Front Immunol. 2011 Oct 12;2:51. doi: 10.3389/fimmu.2011.00051. eCollection 2011. Front Immunol. 2011. PMID: 22566841 Free PMC article.
-
Molecular and epigenetic alterations in normal and malignant myelopoiesis in human leukemia 60 (HL60) promyelocytic cell line model.Front Cell Dev Biol. 2023 Feb 2;11:1060537. doi: 10.3389/fcell.2023.1060537. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36819104 Free PMC article.
References
-
- Kantari C, Pederzoli-Ribeil M, Witko-Sarsat V. The role of neutrophils and monocytes in innate immunity. Contrib Microbiol. 2008;15:118–146. - PubMed
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. - PubMed
-
- Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6(12):1198–1205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

