MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts
- PMID: 19893015
- PMCID: PMC2804031
- DOI: 10.1161/CIRCRESAHA.109.202176
MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts
Abstract
Rationale: MicroRNA (miR)-133a regulates cardiac and skeletal muscle differentiation and plays an important role in cardiac development. Because miR-133a levels decrease during reactive cardiac hypertrophy, some have considered that restoring miR-133a levels could suppress hypertrophic remodeling.
Objective: To prevent the "normal" downregulation of miR-133a induced by an acute hypertrophic stimulus in the adult heart.
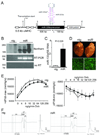
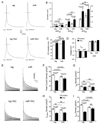
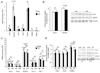
Methods and results: miR-133a is downregulated in transverse aortic constriction (TAC) and isoproterenol-induced hypertrophy, but not in 2 genetic hypertrophy models. Using MYH6 promoter-directed expression of a miR-133a genomic precursor, increased cardiomyocyte miR-133a had no effect on postnatal cardiac development assessed by measures of structure, function, and mRNA profile. However, increased miR-133a levels increased QT intervals in surface electrocardiographic recordings and action potential durations in isolated ventricular myocytes, with a decrease in the fast component of the transient outward K+ current, I(to,f), at baseline. Transgenic expression of miR-133a prevented TAC-associated miR-133a downregulation and improved myocardial fibrosis and diastolic function without affecting the extent of hypertrophy. I(to,f) downregulation normally observed post-TAC was prevented in miR-133a transgenic mice, although action potential duration and QT intervals did not reflect this benefit. miR-133a transgenic hearts had no significant alterations of basal or post-TAC mRNA expression profiles, although decreased mRNA and protein levels were observed for the I(to,f) auxiliary KChIP2 subunit, which is not a predicted target.
Conclusions: These results reveal striking differences between in vitro and in vivo phenotypes of miR expression, and further suggest that mRNA signatures do not reliably predict either direct miR targets or major miR effects.
Figures
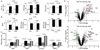

Comment in
-
The role of microRNA-133 in cardiac hypertrophy uncovered.Circ Res. 2010 Jan 8;106(1):16-8. doi: 10.1161/CIRCRESAHA.109.212183. Circ Res. 2010. PMID: 20056941 Free PMC article. No abstract available.
Similar articles
-
The role of microRNA-133 in cardiac hypertrophy uncovered.Circ Res. 2010 Jan 8;106(1):16-8. doi: 10.1161/CIRCRESAHA.109.212183. Circ Res. 2010. PMID: 20056941 Free PMC article. No abstract available.
-
MG53, A Novel Regulator of KChIP2 and Ito,f, Plays a Critical Role in Electrophysiological Remodeling in Cardiac Hypertrophy.Circulation. 2019 Apr 30;139(18):2142-2156. doi: 10.1161/CIRCULATIONAHA.118.029413. Circulation. 2019. PMID: 30760025
-
HDACs Regulate miR-133a Expression in Pressure Overload-Induced Cardiac Fibrosis.Circ Heart Fail. 2015 Nov;8(6):1094-104. doi: 10.1161/CIRCHEARTFAILURE.114.001781. Epub 2015 Sep 14. Circ Heart Fail. 2015. PMID: 26371176 Free PMC article.
-
MicroRNA-133a and Myocardial Infarction.Cell Transplant. 2019 Jul;28(7):831-838. doi: 10.1177/0963689719843806. Epub 2019 Apr 14. Cell Transplant. 2019. PMID: 30983393 Free PMC article. Review.
-
[Research progress on miR-21 in heart diseases].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019 Apr 25;48(2):214-218. doi: 10.3785/j.issn.1008-9292.2019.04.14. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 31309761 Free PMC article. Review. Chinese.
Cited by
-
Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes.Circ Res. 2016 May 27;118(11):1808-29. doi: 10.1161/CIRCRESAHA.116.306923. Circ Res. 2016. PMID: 27230643 Free PMC article. Review.
-
Emerging roles of non-coding RNAs in fibroblast to myofibroblast transition and fibrotic diseases.Front Pharmacol. 2024 Jul 24;15:1423045. doi: 10.3389/fphar.2024.1423045. eCollection 2024. Front Pharmacol. 2024. PMID: 39114349 Free PMC article. Review.
-
Myocardial remodeling, an overview.Heart Fail Rev. 2011 Jan;16(1):1-4. doi: 10.1007/s10741-010-9192-4. Heart Fail Rev. 2011. PMID: 20872278 Review. No abstract available.
-
Editorial: The Non-coding Genome and Cardiovascular Disease.Front Cardiovasc Med. 2019 Jul 16;6:98. doi: 10.3389/fcvm.2019.00098. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 31380396 Free PMC article. No abstract available.
-
The relevance of microRNA in post-infarction left ventricular remodelling and heart failure.Heart Fail Rev. 2019 Jul;24(4):575-586. doi: 10.1007/s10741-019-09770-9. Heart Fail Rev. 2019. PMID: 30710255 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL034161-20/HL/NHLBI NIH HHS/United States
- R01 HL066388-07/HL/NHLBI NIH HHS/United States
- P50 HL077101-050002/HL/NHLBI NIH HHS/United States
- P50-HL077101/HL/NHLBI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R01 HL034161-21/HL/NHLBI NIH HHS/United States
- R01 HL066388-08/HL/NHLBI NIH HHS/United States
- P50 HL077101-03/HL/NHLBI NIH HHS/United States
- R01 HL066388/HL/NHLBI NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- R01-HL034161/HL/NHLBI NIH HHS/United States
- R01 HL034161/HL/NHLBI NIH HHS/United States
- P50 HL077101/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

