Embryonic retinal cells and support to mature retinal neurons
- PMID: 19892872
- PMCID: PMC2868403
- DOI: 10.1167/iovs.09-4447
Embryonic retinal cells and support to mature retinal neurons
Abstract
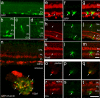
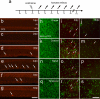
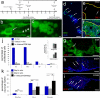
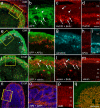
Purpose. There is a paucity of neuron replacement studies for retinal ganglion cells. Given the complex phenotype of these neurons, replacement of ganglion cells may be impossible. However, transplanted embryonic cells could provide factors that promote the survival of these neurons. The authors sought to determine whether transplanted embryonic retinal cells from various stages of development influence the survival of mature ganglion cells Methods. Acutely dissociated retinal cells, obtained from chick embryos, were transplanted into the vitreous chamber of posthatch chicken eyes after the ganglion cells were selectively damaged. Eight days after transplantation, numbers of ganglion cells were determined Results. Embryonic retinal cells from embryonic day (E)7, E10, and E11 promoted the survival of ganglion cells, whereas cells from earlier or later stages of development or from other tissue sources did not. The environment provided by the posthatch eye did not support the proliferation of the embryo-derived cells, unlike the environment provided by culture conditions. Furthermore, cells that migrated into the retina failed to express neuronal or glial markers; those that remained in the vitreous formed aggregates of neuronal and glial cells Conclusions. The environment provided within the mature retina does not support the differentiation and proliferation of retinal progenitors. Furthermore, embryo-derived cells likely produce secreted factors that promote the survival of damaged ganglion cells. Therefore, embryonic retinal cells could be applied as a cell-based survival therapy to treat neurodegenerative diseases of the retina.
Figures
Similar articles
-
Transplantation of cells from eye-like structures differentiated from embryonic stem cells in vitro and in vivo regeneration of retinal ganglion-like cells.Graefes Arch Clin Exp Ophthalmol. 2008 Feb;246(2):255-65. doi: 10.1007/s00417-007-0710-6. Epub 2007 Nov 15. Graefes Arch Clin Exp Ophthalmol. 2008. PMID: 18004585
-
Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate.Invest Ophthalmol Vis Sci. 2007 Jan;48(1):446-54. doi: 10.1167/iovs.06-0190. Invest Ophthalmol Vis Sci. 2007. PMID: 17197566 Free PMC article.
-
Suppression of fibroblast growth factor 2 expression by antisense oligonucleotides inhibits embryonic chick neural retina cell differentiation and survival in vivo.Dev Dyn. 1998 May;212(1):63-74. doi: 10.1002/(SICI)1097-0177(199805)212:1<63::AID-AJA6>3.0.CO;2-0. Dev Dyn. 1998. PMID: 9603424
-
Ganglioside expression during differentiation of chick retinal cells in vitro.Neurochem Res. 2000 Jan;25(1):163-9. doi: 10.1023/a:1007560004244. Neurochem Res. 2000. PMID: 10685616 Review.
-
Transplantation of mammalian embryonic stem cells and their derivatives to avian embryos.Stem Cell Rev Rep. 2010 Sep;6(3):473-83. doi: 10.1007/s12015-010-9161-2. Stem Cell Rev Rep. 2010. PMID: 20533000 Review.
Cited by
-
Neuroprotection by α2-Adrenergic Receptor Stimulation after Excitotoxic Retinal Injury: A Study of the Total Population of Retinal Ganglion Cells and Their Distribution in the Chicken Retina.PLoS One. 2016 Sep 9;11(9):e0161862. doi: 10.1371/journal.pone.0161862. eCollection 2016. PLoS One. 2016. PMID: 27611432 Free PMC article.
-
NF-κB signaling regulates the formation of proliferating Müller glia-derived progenitor cells in the avian retina.Development. 2020 May 22;147(10):dev183418. doi: 10.1242/dev.183418. Development. 2020. PMID: 32291273 Free PMC article.
-
A Novel Scoring System Approach to Assess Patients with Lyme Disease (Nutech Functional Score).J Glob Infect Dis. 2018 Jan-Mar;10(1):3-6. doi: 10.4103/jgid.jgid_11_17. J Glob Infect Dis. 2018. PMID: 29563715 Free PMC article.
-
A comparative analysis of Müller glia-mediated regeneration in the vertebrate retina.Exp Eye Res. 2014 Jun;123:121-30. doi: 10.1016/j.exer.2013.06.019. Epub 2013 Jul 9. Exp Eye Res. 2014. PMID: 23851023 Free PMC article. Review.
-
Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages.J Exp Med. 2011 Jan 17;208(1):23-39. doi: 10.1084/jem.20101202. Epub 2011 Jan 10. J Exp Med. 2011. PMID: 21220455 Free PMC article.
References
-
- MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature 2006;444:203–207 - PubMed
-
- Seiler MJ, Aramant RB. Transplantation of neuroblastic progenitor cells as a sheet preserves and restores retinal function. Semin Ophthalmol 2005;20:31–42 - PubMed
-
- Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog Retin Eye Res 2004;23:475–494 - PubMed
-
- Arai S, Thomas BB, Seiler MJ, et al. Restoration of visual responses following transplantation of intact retinal sheets in rd mice. Exp Eye Res 2004;79:331–341 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

