The vacuole import and degradation pathway utilizes early steps of endocytosis and actin polymerization to deliver cargo proteins to the vacuole for degradation
- PMID: 19892709
- PMCID: PMC2801277
- DOI: 10.1074/jbc.M109.028241
The vacuole import and degradation pathway utilizes early steps of endocytosis and actin polymerization to deliver cargo proteins to the vacuole for degradation
Abstract
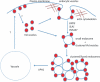
When glucose is added to yeast cells that are starved for 3 days, fructose-1,6-bisphosphatase (FBPase) and malate dehydrogenase 2 are degraded in the vacuole via the vacuole import and degradation (Vid) pathway. In this study, we examined the distribution of FBPase at the ultrastructural level. FBPase was observed in areas close to the plasma membrane and in cytoplasmic structures that are heterogeneous in size and density. We have isolated these intracellular structures that contain FBPase, the Vid vesicle marker Vid24p, and the endosomal marker Pep12p. They appeared irregular in size and shape. In yeast, actin polymerization plays an important role in early steps of endocytosis. Mutants that affect actin polymerization inhibited FBPase degradation, suggesting that actin polymerization is important for FBPase degradation. Both FBPase and malate dehydrogenase 2 were associated with actin patches. Vid vesicle proteins such as Vid24p or Sec28p were also at actin patches, although they dissociated from these structures at later time points. We propose that Vid24p and Sec28p are present at actin patches during glucose starvation. Cargo proteins arrive at these sites following the addition of glucose, and the endocytic vesicles then pinch off from the plasma membrane. Following the fusion of endosomes with the vacuole, cargo proteins are then degraded in the vacuole.
Figures
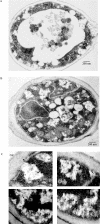

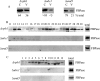

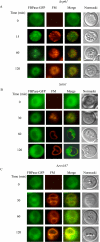
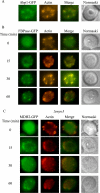
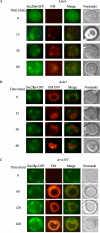
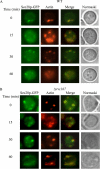
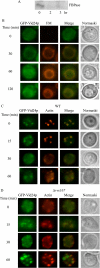
Similar articles
-
Vps34p is required for the decline of extracellular fructose-1,6-bisphosphatase in the vacuole import and degradation pathway.J Biol Chem. 2012 Sep 21;287(39):33080-93. doi: 10.1074/jbc.M112.360412. Epub 2012 Jul 25. J Biol Chem. 2012. PMID: 22833678 Free PMC article.
-
The vacuolar import and degradation pathway merges with the endocytic pathway to deliver fructose-1,6-bisphosphatase to the vacuole for degradation.J Biol Chem. 2008 Sep 19;283(38):26116-27. doi: 10.1074/jbc.M709922200. Epub 2008 Jul 25. J Biol Chem. 2008. PMID: 18660504 Free PMC article.
-
Vid30 is required for the association of Vid vesicles and actin patches in the vacuole import and degradation pathway.Autophagy. 2012 Jan;8(1):29-46. doi: 10.4161/auto.8.1.18104. Epub 2012 Jan 1. Autophagy. 2012. PMID: 22082961 Free PMC article.
-
Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces cerevisiae.Membranes (Basel). 2014 Sep 3;4(3):608-29. doi: 10.3390/membranes4030608. Membranes (Basel). 2014. PMID: 25192542 Free PMC article. Review.
-
The key gluconeogenic enzyme fructose-1,6-bisphosphatase is secreted during prolonged glucose starvation and is internalized following glucose re-feeding via the non-classical secretory and internalizing pathways in Saccharomyces cerevisiae.Plant Signal Behav. 2013 Aug;8(8):e24936. doi: 10.4161/psb.24936. Epub 2013 Jun 26. Plant Signal Behav. 2013. PMID: 23673352 Free PMC article. Review.
Cited by
-
A GID E3 ligase assembly ubiquitinates an Rsp5 E3 adaptor and regulates plasma membrane transporters.EMBO Rep. 2022 Jun 7;23(6):e53835. doi: 10.15252/embr.202153835. Epub 2022 Apr 19. EMBO Rep. 2022. PMID: 35437932 Free PMC article.
-
The endocytosis gene END3 is essential for the glucose-induced rapid decline of small vesicles in the extracellular fraction in Saccharomyces cerevisiae.J Extracell Vesicles. 2014 Mar 21;3. doi: 10.3402/jev.v3.23497. eCollection 2014. J Extracell Vesicles. 2014. PMID: 24665361 Free PMC article.
-
Comparative proteomic analysis of transition of saccharomyces cerevisiae from glucose-deficient medium to glucose-rich medium.Proteome Sci. 2012 Jun 12;10(1):40. doi: 10.1186/1477-5956-10-40. Proteome Sci. 2012. PMID: 22691627 Free PMC article.
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
-
A network-based approach on elucidating the multi-faceted nature of chronological aging in S. cerevisiae.PLoS One. 2011;6(12):e29284. doi: 10.1371/journal.pone.0029284. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216232 Free PMC article.
References
-
- Goldberg A. L. (2007) Biochem. Soc. Trans. 35, 12–17 - PubMed
-
- Varshavsky A. (2005) Trends Biochem. Sci. 30, 283–286 - PubMed
-
- Seglen P. O., Gordon P. B., Holen I. (1990) Semin. Cell Biol. 1, 441–448 - PubMed
-
- Neely A. N., Cox J. R., Fortney J. A., Schworer C. M., Mortimore G. E. (1977) J. Biol. Chem. 252, 6948–6954 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

