Retinoic acid from the meninges regulates cortical neuron generation
- PMID: 19879845
- PMCID: PMC2772834
- DOI: 10.1016/j.cell.2009.10.004
Retinoic acid from the meninges regulates cortical neuron generation
Erratum in
- Cell. 2011 Aug 5;146(3):486
Abstract
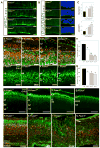
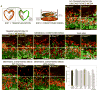
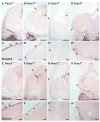
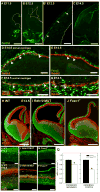
Extrinsic signals controlling generation of neocortical neurons during embryonic life have been difficult to identify. In this study we demonstrate that the dorsal forebrain meninges communicate with the adjacent radial glial endfeet and influence cortical development. We took advantage of Foxc1 mutant mice with defects in forebrain meningeal formation. Foxc1 dosage and loss of meninges correlated with a dramatic reduction in both neuron and intermediate progenitor production and elongation of the neuroepithelium. Several types of experiments demonstrate that retinoic acid (RA) is the key component of this secreted activity. In addition, Rdh10- and Raldh2-expressing cells in the dorsal meninges were either reduced or absent in the Foxc1 mutants, and Rdh10 mutants had a cortical phenotype similar to the Foxc1 null mutants. Lastly, in utero RA treatment rescued the cortical phenotype in Foxc1 mutants. These results establish RA as a potent, meningeal-derived cue required for successful corticogenesis.
Figures


Similar articles
-
Cerebrovascular defects in Foxc1 mutants correlate with aberrant WNT and VEGF-A pathways downstream of retinoic acid from the meninges.Dev Biol. 2016 Dec 1;420(1):148-165. doi: 10.1016/j.ydbio.2016.09.019. Epub 2016 Sep 23. Dev Biol. 2016. PMID: 27671872 Free PMC article.
-
Investigation of retinoic acid function during embryonic brain development using retinaldehyde-rescued Rdh10 knockout mice.Dev Dyn. 2013 Sep;242(9):1056-65. doi: 10.1002/dvdy.23999. Epub 2013 Jul 22. Dev Dyn. 2013. PMID: 23765990 Free PMC article.
-
CoupTFI interacts with retinoic acid signaling during cortical development.PLoS One. 2013;8(3):e58219. doi: 10.1371/journal.pone.0058219. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472160 Free PMC article.
-
Forebrain induction, retinoic acid, and vulnerability to schizophrenia: insights from molecular and genetic analysis in developing mice.Biol Psychiatry. 1999 Jul 1;46(1):19-30. doi: 10.1016/s0006-3223(99)00002-5. Biol Psychiatry. 1999. PMID: 10394471 Review.
-
[Genetic control of the development by retinoic acid].C R Seances Soc Biol Fil. 1997;191(1):77-90. C R Seances Soc Biol Fil. 1997. PMID: 9181129 Review. French.
Cited by
-
Post-transcriptional regulatory elements and spatiotemporal specification of neocortical stem cells and projection neurons.Neuroscience. 2013 Sep 17;248:499-528. doi: 10.1016/j.neuroscience.2013.05.042. Epub 2013 May 30. Neuroscience. 2013. PMID: 23727006 Free PMC article. Review.
-
Activin induces cortical interneuron identity and differentiation in embryonic stem cell-derived telencephalic neural precursors.Nat Commun. 2012 May 15;3:841. doi: 10.1038/ncomms1817. Nat Commun. 2012. PMID: 22588303
-
Mechanisms of retinoic acid signalling and its roles in organ and limb development.Nat Rev Mol Cell Biol. 2015 Feb;16(2):110-23. doi: 10.1038/nrm3932. Epub 2015 Jan 5. Nat Rev Mol Cell Biol. 2015. PMID: 25560970 Free PMC article. Review.
-
'Sealing off the CNS': cellular and molecular regulation of blood-brain barriergenesis.Curr Opin Neurobiol. 2013 Dec;23(6):1057-64. doi: 10.1016/j.conb.2013.06.006. Epub 2013 Jul 15. Curr Opin Neurobiol. 2013. PMID: 23867075 Free PMC article. Review.
-
Morphological defects in a novel Rdh10 mutant that has reduced retinoic acid biosynthesis and signaling.Genesis. 2012 May;50(5):415-23. doi: 10.1002/dvg.22002. Epub 2012 Jan 25. Genesis. 2012. PMID: 22162152 Free PMC article.
References
-
- Anchan RM, Drake DP, Haines CF, Gerwe EA, LaMantia AS. Disruption of local retinoid-mediated gene expression accompanies abnormal development in the mammalian olfactory pathway. J Comp Neurol. 1997;379:171–184. - PubMed
-
- Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. - PubMed
-
- Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. - PubMed
-
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

