T helper 17 cells promote cytotoxic T cell activation in tumor immunity
- PMID: 19879162
- PMCID: PMC2787786
- DOI: 10.1016/j.immuni.2009.09.014
T helper 17 cells promote cytotoxic T cell activation in tumor immunity
Abstract
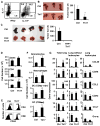
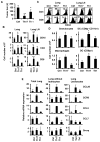
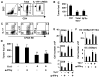
Although T helper 17 (Th17) cells have been found in tumor tissues, their function in cancer immunity is unclear. We found that interleukin-17A (IL-17A)-deficient mice were more susceptible to developing lung melanoma. Conversely, adoptive T cell therapy with tumor-specific Th17 cells prevented tumor development. Importantly, the Th17 cells retained their cytokine signature and exhibited stronger therapeutic efficacy than Th1 cells. Unexpectedly, therapy using Th17 cells elicited a remarkable activation of tumor-specific CD8(+) T cells, which were necessary for the antitumor effect. Th17 cells promoted dendritic cell recruitment into the tumor tissues and in draining lymph nodes increased CD8 alpha(+) dendritic cells containing tumor material. Moreover, Th17 cells promoted CCL20 chemokine production by tumor tissues, and tumor-bearing CCR6-deficient mice did not respond to Th17 cell therapy. Thus, Th17 cells elicited a protective inflammation that promotes the activation of tumor-specific CD8(+) T cells. These findings have important implications in antitumor immunotherapies.
Figures

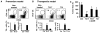
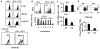
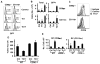
Comment in
-
T helper 17 T cells do good for cancer immunotherapy.Immunotherapy. 2010 Jan;2(1):21-4. doi: 10.2217/imt.09.83. Immunotherapy. 2010. PMID: 20635888
Similar articles
-
Th9 cells promote antitumor immune responses in vivo.J Clin Invest. 2012 Nov;122(11):4160-71. doi: 10.1172/JCI65459. Epub 2012 Oct 15. J Clin Invest. 2012. PMID: 23064366 Free PMC article.
-
Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes.Blood. 2009 Jun 11;113(24):6102-11. doi: 10.1182/blood-2008-12-195354. Epub 2009 Apr 14. Blood. 2009. PMID: 19366986 Free PMC article.
-
IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines.J Leukoc Biol. 2009 Aug;86(2):435-43. doi: 10.1189/JLB.0109046. J Leukoc Biol. 2009. PMID: 19487306
-
Translational mini-review series on Th17 cells: are T helper 17 cells really pathogenic in autoimmunity?Clin Exp Immunol. 2010 Feb;159(2):131-6. doi: 10.1111/j.1365-2249.2009.04039.x. Epub 2009 Nov 11. Clin Exp Immunol. 2010. PMID: 19912250 Free PMC article. Review.
-
Migration and function of Th17 cells.Inflamm Allergy Drug Targets. 2009 Jul;8(3):221-8. doi: 10.2174/187152809788681001. Inflamm Allergy Drug Targets. 2009. PMID: 19601882 Review.
Cited by
-
Transcriptional profiling of disease-induced host responses in bovine tuberculosis and the identification of potential diagnostic biomarkers.PLoS One. 2012;7(2):e30626. doi: 10.1371/journal.pone.0030626. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359547 Free PMC article.
-
IL-17A Drives Oxidative Stress and Cell Growth in A549 Lung Epithelial Cells: Potential Protective Action of Oleuropein.Nutrients. 2024 Jul 3;16(13):2123. doi: 10.3390/nu16132123. Nutrients. 2024. PMID: 38999871 Free PMC article.
-
Interleukin-17 and type 17 helper T cells in cancer management and research.Immunotargets Ther. 2014 Mar 10;3:39-54. doi: 10.2147/ITT.S56529. eCollection 2014. Immunotargets Ther. 2014. PMID: 27471699 Free PMC article. Review.
-
A biomimetic yeast shell vaccine coated with layered double hydroxides induces a robust humoral and cellular immune response against tumors.Nanoscale Adv. 2020 Jun 26;2(8):3494-3506. doi: 10.1039/d0na00249f. eCollection 2020 Aug 11. Nanoscale Adv. 2020. PMID: 36134256 Free PMC article.
-
Natural CD4+ T-cell responses against indoleamine 2,3-dioxygenase.PLoS One. 2012;7(4):e34568. doi: 10.1371/journal.pone.0034568. Epub 2012 Apr 23. PLoS One. 2012. PMID: 22539948 Free PMC article.
References
-
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. - PubMed
-
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. - PubMed
-
- Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. - PubMed
-
- Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

