In AbetaPP-overexpressing cultured human muscle fibers proteasome inhibition enhances phosphorylation of AbetaPP751 and GSK3beta activation: effects mitigated by lithium and apparently relevant to sporadic inclusion-body myositis
- PMID: 19878439
- PMCID: PMC2809129
- DOI: 10.1111/j.1471-4159.2009.06461.x
In AbetaPP-overexpressing cultured human muscle fibers proteasome inhibition enhances phosphorylation of AbetaPP751 and GSK3beta activation: effects mitigated by lithium and apparently relevant to sporadic inclusion-body myositis
Abstract
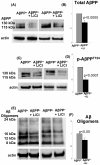
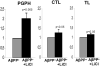
Muscle fiber degeneration in sporadic inclusion-body myositis (s-IBM) is characterized by accumulation of multiprotein aggregates, including aggregated amyloid-beta (Abeta)-precursor protein 751 (AbetaPP751), Abeta, phosphorylated tau, and other 'Alzheimer-characteristic' proteins. Proteasome inhibition is an important component of the s-IBM pathogenesis. In brains of Alzheimer's disease (AD) patients and AD transgenic-mouse models, phosphorylation of neuronal AbetaPP695 (p-AbetaPP) on Thr668 (equivalent to T724 of AbetaPP751) is considered detrimental because it increases generation of cytotoxic Abeta and induces tau phosphorylation. Activated glycogen synthase kinase3beta (GSK3beta) is involved in phosphorylation of both AbetaPP and tau. Lithium, an inhibitor of GSK3beta, was reported to reduce levels of both the total AbetaPP and p-AbetaPP in AD animal models. In relation to s-IBM, we now show for the first time that (1) In AbetaPP-overexpressing cultured human muscle fibers (human muscle culture IBM model: (a) proteasome inhibition significantly increases GSK3beta activity and AbetaPP phosphorylation, (b) treatment with lithium decreases (i) phosphorylated-AbetaPP, (ii) total amount of AbetaPP, (iii) Abeta oligomers, and (iv) GSK3beta activity; and (c) lithium improves proteasome function. (2) In biopsied s-IBM muscle fibers, GSK3beta is significantly activated and AbetaPP is phosphorylated on Thr724. Accordingly, treatment with lithium, or other GSK3beta inhibitors, might benefit s-IBM patients.
Figures





Similar articles
-
Proteasome inhibition and aggresome formation in sporadic inclusion-body myositis and in amyloid-beta precursor protein-overexpressing cultured human muscle fibers.Am J Pathol. 2005 Aug;167(2):517-26. doi: 10.1016/s0002-9440(10)62994-x. Am J Pathol. 2005. PMID: 16049336 Free PMC article.
-
Homocysteine-induced endoplasmic reticulum protein (Herp) is up-regulated in sporadic inclusion-body myositis and in endoplasmic reticulum stress-induced cultured human muscle fibers.J Neurochem. 2006 Mar;96(5):1491-9. doi: 10.1111/j.1471-4159.2006.03668.x. Epub 2006 Jan 25. J Neurochem. 2006. PMID: 16441512
-
AbetaPP-overexpression and proteasome inhibition increase alphaB-crystallin in cultured human muscle: relevance to inclusion-body myositis.Neuromuscul Disord. 2006 Dec;16(12):839-44. doi: 10.1016/j.nmd.2006.08.009. Epub 2006 Oct 23. Neuromuscul Disord. 2006. PMID: 17056255 Free PMC article.
-
Sporadic inclusion-body myositis: conformational multifactorial ageing-related degenerative muscle disease associated with proteasomal and lysosomal inhibition, endoplasmic reticulum stress, and accumulation of amyloid-β42 oligomers and phosphorylated tau.Presse Med. 2011 Apr;40(4 Pt 2):e219-35. doi: 10.1016/j.lpm.2010.11.024. Epub 2011 Mar 9. Presse Med. 2011. PMID: 21392932 Review.
-
Pathogenic considerations in sporadic inclusion-body myositis, a degenerative muscle disease associated with aging and abnormalities of myoproteostasis.J Neuropathol Exp Neurol. 2012 Aug;71(8):680-93. doi: 10.1097/NEN.0b013e31826183c8. J Neuropathol Exp Neurol. 2012. PMID: 22805774 Review.
Cited by
-
GSK-3 in Neurodegenerative Diseases.Int J Alzheimers Dis. 2011;2011:189246. doi: 10.4061/2011/189246. Epub 2011 May 4. Int J Alzheimers Dis. 2011. PMID: 21629738 Free PMC article.
-
Lithium chloride attenuates cell death in oculopharyngeal muscular dystrophy by perturbing Wnt/β-catenin pathway.Cell Death Dis. 2013 Oct 3;4(10):e821. doi: 10.1038/cddis.2013.342. Cell Death Dis. 2013. PMID: 24091664 Free PMC article.
-
Immune and myodegenerative pathomechanisms in inclusion body myositis.Ann Clin Transl Neurol. 2017 May 16;4(6):422-445. doi: 10.1002/acn3.419. eCollection 2017 Jun. Ann Clin Transl Neurol. 2017. PMID: 28589170 Free PMC article. Review.
-
Impaired autophagy in sporadic inclusion-body myositis and in endoplasmic reticulum stress-provoked cultured human muscle fibers.Am J Pathol. 2010 Sep;177(3):1377-87. doi: 10.2353/ajpath.2010.100050. Epub 2010 Jul 8. Am J Pathol. 2010. PMID: 20616343 Free PMC article.
-
Updates on the Immunopathology in Idiopathic Inflammatory Myopathies.Curr Rheumatol Rep. 2021 Jul 1;23(7):56. doi: 10.1007/s11926-021-01017-7. Curr Rheumatol Rep. 2021. PMID: 34212266 Review.
References
-
- Anderton BH, Betts J, Blackstock WP, et al. Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp. 2001;67:73–80. - PubMed
-
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J. Biol. Chem. 2001;276:40353–40361. - PubMed
-
- Aplin AE, Gibb GM, Jacobsen JS, Gallo JM, Anderton BH. In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3beta. J. Neurochem. 1996;67:699–707. - PubMed
-
- Askanas V, Engel WK. Cultured normal and genetically abnormal human muscle. In: Rowland LP, Di Mauro S, editors. The Handbook of Clinical Neurology, Myopathies. Vol. 18. North Holland; Amsterdam: 1992. pp. 85–116.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

