S100A12 mediates aortic wall remodeling and aortic aneurysm
- PMID: 19875725
- PMCID: PMC2878187
- DOI: 10.1161/CIRCRESAHA.109.209486
S100A12 mediates aortic wall remodeling and aortic aneurysm
Abstract
Rationale: S100A12 is a small calcium binding protein that is a ligand of RAGE (receptor for advanced glycation end products). RAGE has been extensively implicated in inflammatory states such as atherosclerosis, but the role of S100A12 as its ligand is less clear.
Objective: To test the role of S100A12 in vascular inflammation, we generated and analyzed mice expressing human S100A12 in vascular smooth muscle under control of the smooth muscle 22alpha promoter because S100A12 is not present in mice.
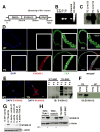
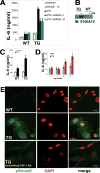
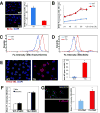
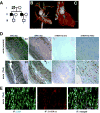
Methods and results: Transgenic mice displayed pathological vascular remodeling with aberrant thickening of the aortic media, disarray of elastic fibers, and increased collagen deposition, together with increased latent matrix metalloproteinase-2 protein and reduction in smooth muscle stress fibers leading to a progressive dilatation of the aorta. In primary aortic smooth muscle cell cultures, we found that S100A12 mediates increased interleukin-6 production, activation of transforming growth factor beta pathways and increased metabolic activity with enhanced oxidative stress. To correlate our findings to human aortic aneurysmal disease, we examined S100A12 expression in aortic tissue from patients with thoracic aortic aneurysm and found increased S100A12 expression in vascular smooth muscle cells.
Conclusions: S100A12 expression is sufficient to activate pathogenic pathways through the modulation of oxidative stress, inflammation and vascular remodeling in vivo.
Figures



Comment in
-
S100A12 links to thoracic aortic aneurysms.Circ Res. 2010 Jan 8;106(1):13-5. doi: 10.1161/CIRCRESAHA.109.210757. Circ Res. 2010. PMID: 20056940 Free PMC article. No abstract available.
Similar articles
-
S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2496-507. doi: 10.1161/ATVBAHA.115.302072. Epub 2015 Oct 29. Arterioscler Thromb Vasc Biol. 2015. PMID: 26515415 Free PMC article. Review.
-
S100A12 links to thoracic aortic aneurysms.Circ Res. 2010 Jan 8;106(1):13-5. doi: 10.1161/CIRCRESAHA.109.210757. Circ Res. 2010. PMID: 20056940 Free PMC article. No abstract available.
-
S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications.J Am Coll Cardiol. 2012 Aug 21;60(8):775-85. doi: 10.1016/j.jacc.2012.04.027. Epub 2012 Jul 18. J Am Coll Cardiol. 2012. PMID: 22818064 Free PMC article.
-
Beneficial effects of quinoline-3-carboxamide (ABR-215757) on atherosclerotic plaque morphology in S100A12 transgenic ApoE null mice.Atherosclerosis. 2013 May;228(1):69-79. doi: 10.1016/j.atherosclerosis.2013.02.023. Epub 2013 Feb 28. Atherosclerosis. 2013. PMID: 23497784 Free PMC article.
-
S100 proteins in atherosclerosis.Clin Chim Acta. 2020 Mar;502:293-304. doi: 10.1016/j.cca.2019.11.019. Epub 2019 Nov 30. Clin Chim Acta. 2020. PMID: 31794767 Review.
Cited by
-
IL-22 is induced by S100/calgranulin and impairs cholesterol efflux in macrophages by downregulating ABCG1.J Lipid Res. 2014 Mar;55(3):443-54. doi: 10.1194/jlr.M044305. Epub 2013 Dec 23. J Lipid Res. 2014. PMID: 24367046 Free PMC article.
-
Accentuating and Opposing Factors Leading to Development of Thoracic Aortic Aneurysms Not Due to Genetic or Inherited Conditions.Front Cardiovasc Med. 2015 May 26;2:21. doi: 10.3389/fcvm.2015.00021. eCollection 2015. Front Cardiovasc Med. 2015. PMID: 26664893 Free PMC article. Review.
-
S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2496-507. doi: 10.1161/ATVBAHA.115.302072. Epub 2015 Oct 29. Arterioscler Thromb Vasc Biol. 2015. PMID: 26515415 Free PMC article. Review.
-
Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections.Circ Res. 2015 Apr 10;116(8):1448-61. doi: 10.1161/CIRCRESAHA.114.304936. Circ Res. 2015. PMID: 25858068 Free PMC article. Review.
-
Genetic pathways of vascular calcification.Trends Cardiovasc Med. 2012 May;22(4):93-8. doi: 10.1016/j.tcm.2012.07.002. Trends Cardiovasc Med. 2012. PMID: 23040839 Free PMC article. Review.
References
-
- Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. - PubMed
-
- Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, Lalla E, Chitnis S, Monteiro J, Stickland MH, Bucciarelli LG, Moser B, Moxley G, Itescu S, Grant PJ, Gregersen PK, Stern DM, Schmidt AM. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–135. - PubMed
-
- Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, Fitzgerald O, Roth J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003;42:1383–1389. - PubMed
-
- Yang Z, Yan WX, Cai H, Tedla N, Armishaw C, Di Girolamo N, Wang HW, Hampartzoumian T, Simpson JL, Gibson PG, Hunt J, Hart P, Hughes JM, Perry MA, Alewood PF, Geczy CL. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol. 2007;119:106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL090917-02/HL/NHLBI NIH HHS/United States
- R01 HL078926-05/HL/NHLBI NIH HHS/United States
- K08 HL090917-01A1/HL/NHLBI NIH HHS/United States
- K08 HL080082-04/HL/NHLBI NIH HHS/United States
- F32 HL093992-01/HL/NHLBI NIH HHS/United States
- T32 HL007381/HL/NHLBI NIH HHS/United States
- K08 HL080082/HL/NHLBI NIH HHS/United States
- K08-HL090917/HL/NHLBI NIH HHS/United States
- HL078926/HL/NHLBI NIH HHS/United States
- F32HL93992/HL/NHLBI NIH HHS/United States
- K08 HL090917/HL/NHLBI NIH HHS/United States
- T32HL7381/HL/NHLBI NIH HHS/United States
- R01 HL078926/HL/NHLBI NIH HHS/United States
- K08-HL080082/HL/NHLBI NIH HHS/United States
- F32 HL093992/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

