Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson's disease
- PMID: 19864567
- PMCID: PMC2862566
- DOI: 10.1523/JNEUROSCI.4144-09.2009
Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson's disease
Abstract
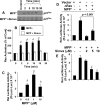
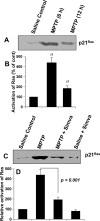
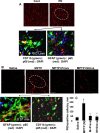
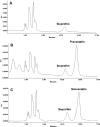
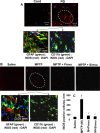
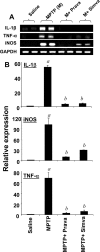
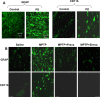
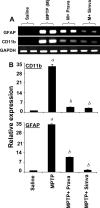
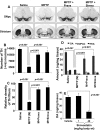
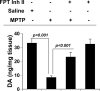
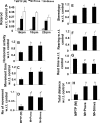
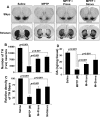
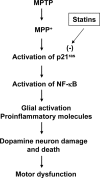
Parkinson's disease (PD) is second only to Alzheimer's disease as the most common devastating human neurodegenerative disorder. Despite intense investigation, no interdictive therapy is available for PD. We investigated whether simvastatin, a Food and Drug Administration-approved cholesterol-lowering drug, could protect against nigrostriatal degeneration after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication to model PD in mice. First, MPP(+) induced the activation of p21(ras) and nuclear factor-kappaB (NF-kappaB) in mouse microglial cells. Inhibition of MPP(+)-induced activation of NF-kappaB by Deltap21(ras), a dominant-negative mutant of p21(ras), supported the involvement of p21(ras) in MPP(+)-induced microglial activation of NF-kappaB. Interestingly, simvastatin attenuated activation of both p21(ras) and NF-kappaB in MPP(+)-stimulated microglial cells. Consistently, we found a very rapid activation of p21(ras) in vivo in the substantia nigra pars compacta of MPTP-intoxicated mice. However, after oral administration, simvastatin entered into the nigra, reduced nigral activation of p21(ras), attenuated nigral activation of NF-kappaB, inhibited nigral expression of proinflammatory molecules, and suppressed nigral activation of glial cells. These findings paralleled dopaminergic neuronal protection, normalized striatal neurotransmitters, and improved motor functions in MPTP-intoxicated mice. Similarly, pravastatin, another cholesterol-lowering drug, suppressed microglial inflammatory responses and protected dopaminergic neurons in MPTP-intoxicated mice, but at levels less than simvastatin. Furthermore, both the statins administered 2 d after initiation of the disease were still capable of inhibiting the demise of dopaminergic neurons and concomitant loss of neurotransmitters, suggesting that statins are capable of slowing down the progression of neuronal loss in the MPTP mouse model. Therefore, we conclude that statins may be of therapeutic benefit for PD patients.
Figures
Similar articles
-
Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson's disease.PLoS One. 2012;7(6):e38113. doi: 10.1371/journal.pone.0038113. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723850 Free PMC article.
-
Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease.Proc Natl Acad Sci U S A. 2007 Nov 20;104(47):18754-9. doi: 10.1073/pnas.0704908104. Epub 2007 Nov 13. Proc Natl Acad Sci U S A. 2007. PMID: 18000063 Free PMC article.
-
Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson's disease.J Ethnopharmacol. 2015 Apr 22;164:247-55. doi: 10.1016/j.jep.2015.01.042. Epub 2015 Feb 7. J Ethnopharmacol. 2015. PMID: 25666429
-
Neuropharmacological approach against MPTP (1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine)-induced mouse model of Parkinson's disease.Acta Neurobiol Exp (Wars). 2011;71(2):269-80. doi: 10.55782/ane-2011-1847. Acta Neurobiol Exp (Wars). 2011. PMID: 21731080 Review.
-
The lesion of the rat substantia nigra pars compacta dopaminergic neurons as a model for Parkinson's disease memory disabilities.Cell Mol Neurobiol. 2002 Jun;22(3):227-37. doi: 10.1023/a:1020736131907. Cell Mol Neurobiol. 2002. PMID: 12469866 Review.
Cited by
-
Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson's disease.PLoS One. 2012;7(6):e38113. doi: 10.1371/journal.pone.0038113. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723850 Free PMC article.
-
Organophosphate pesticide chlorpyrifos impairs STAT1 signaling to induce dopaminergic neurotoxicity: Implications for mitochondria mediated oxidative stress signaling events.Neurobiol Dis. 2018 Sep;117:82-113. doi: 10.1016/j.nbd.2018.05.019. Epub 2018 May 31. Neurobiol Dis. 2018. PMID: 29859868 Free PMC article.
-
Liver X receptors and cholesterol metabolism: role in ventral midbrain development and neurodegeneration.F1000Prime Rep. 2015 Apr 2;7:37. doi: 10.12703/P7-37. eCollection 2015. F1000Prime Rep. 2015. PMID: 26097711 Free PMC article. Review.
-
Neuroprotective and Neurorescue Mode of Action of Bacopa monnieri (L.) Wettst in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinson's Disease: An In Silico and In Vivo Study.Front Pharmacol. 2021 Mar 16;12:616413. doi: 10.3389/fphar.2021.616413. eCollection 2021. Front Pharmacol. 2021. PMID: 33796021 Free PMC article.
-
A Novel Compound ITC-3 Activates the Nrf2 Signaling and Provides Neuroprotection in Parkinson's Disease Models.Neurotox Res. 2015 Nov;28(4):332-45. doi: 10.1007/s12640-015-9550-z. Epub 2015 Aug 2. Neurotox Res. 2015. PMID: 26233727
References
-
- Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M. IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson's disease. Biomed Pharmacother. 1999;53:141–145. - PubMed
-
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
