Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain
- PMID: 19864432
- PMCID: PMC2804222
- DOI: 10.1074/jbc.M109.052548
Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain
Abstract
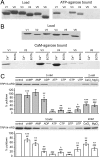
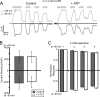
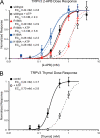
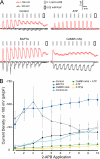
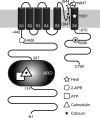
Transient receptor potential vanilloid (TRPV) channels, which include the thermosensitive TRPV1-V4, have large cytoplasmic regions flanking the transmembrane domain, including an N-terminal ankyrin repeat domain. We show that a multiligand binding site for ATP and calmodulin previously identified in the TRPV1 ankyrin repeat domain is conserved in TRPV3 and TRPV4, but not TRPV2. Accordingly, TRPV2 is insensitive to intracellular ATP, while, as previously observed with TRPV1, a sensitizing effect of ATP on TRPV4 required an intact binding site. In contrast, ATP reduced TRPV3 sensitivity and potentiation by repeated agonist stimulations. Thus, ATP and calmodulin, acting through this conserved binding site, are key players in generating the different sensitivity and adaptation profiles of TRPV1, TRPV3, and TRPV4. Our results suggest that competing interactions of ATP and calmodulin influence channel sensitivity to fluctuations in calcium concentration and perhaps even metabolic state. Different feedback mechanisms likely arose because of the different physiological stimuli or temperature thresholds of these channels.
Figures


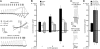
Similar articles
-
Insights into the roles of conserved and divergent residues in the ankyrin repeats of TRPV ion channels.Channels (Austin). 2007 May-Jun;1(3):148-51. doi: 10.4161/chan.4716. Epub 2007 Jul 9. Channels (Austin). 2007. PMID: 18690026
-
Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels.Biochemistry. 2008 Feb 26;47(8):2476-84. doi: 10.1021/bi702109w. Epub 2008 Jan 31. Biochemistry. 2008. PMID: 18232717 Free PMC article.
-
The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity.Neuron. 2007 Jun 21;54(6):905-18. doi: 10.1016/j.neuron.2007.05.027. Neuron. 2007. PMID: 17582331
-
Vanilloid transient receptor potential cation channels: an overview.Curr Pharm Des. 2008;14(1):18-31. doi: 10.2174/138161208783330763. Curr Pharm Des. 2008. PMID: 18220815 Review.
-
Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review.
Cited by
-
Neuropathy-causing TRPV4 mutations disrupt TRPV4-RhoA interactions and impair neurite extension.Nat Commun. 2021 Mar 4;12(1):1444. doi: 10.1038/s41467-021-21699-y. Nat Commun. 2021. PMID: 33664271 Free PMC article.
-
Crystal structure of the epithelial calcium channel TRPV6.Nature. 2016 Jun 23;534(7608):506-11. doi: 10.1038/nature17975. Epub 2016 Jun 13. Nature. 2016. PMID: 27296226 Free PMC article.
-
The novel high-frequency variant of TRPV3 p.A628T in East Asians showing faster sensitization in response to chemical agonists.Pflugers Arch. 2019 Oct;471(10):1273-1289. doi: 10.1007/s00424-019-02309-9. Epub 2019 Oct 14. Pflugers Arch. 2019. PMID: 31612282
-
Transient receptor potential ion-channel subfamily V member 4: a potential target for cancer treatment.Cell Death Dis. 2019 Jun 24;10(7):497. doi: 10.1038/s41419-019-1708-9. Cell Death Dis. 2019. PMID: 31235786 Free PMC article. Review.
-
Recognition of 2',5'-linked oligoadenylates by human ribonuclease L: molecular dynamics study.J Mol Model. 2014 Apr;20(4):2123. doi: 10.1007/s00894-014-2123-x. Epub 2014 Mar 16. J Mol Model. 2014. PMID: 24633766
References
-
- Nijenhuis T., Hoenderop J. G., Bindels R. J. (2005) Pflugers Arch. 451, 181–192 - PubMed
-
- Caterina M. J. (2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R64–R76 - PubMed
-
- Tominaga M., Caterina M. J. (2004) J. Neurobiol. 61, 3–12 - PubMed
-
- Xu H., Delling M., Jun J. C., Clapham D. E. (2006) Nat. Neurosci. 9, 628–635 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

