Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury
- PMID: 19846712
- PMCID: PMC2801423
- DOI: 10.1523/JNEUROSCI.3376-09.2009
Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury
Abstract
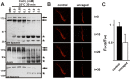
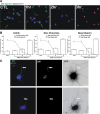
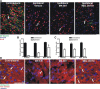
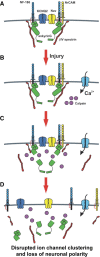
Many factors contribute to nervous system dysfunction and failure to regenerate after injury or disease. Here, we describe a previously unrecognized mechanism for nervous system injury. We show that neuronal injury causes rapid, irreversible, and preferential proteolysis of the axon initial segment (AIS) cytoskeleton independently of cell death or axon degeneration, leading to loss of both ion channel clusters and neuronal polarity. Furthermore, we show this is caused by proteolysis of the AIS cytoskeletal proteins ankyrinG and betaIV spectrin by the calcium-dependent cysteine protease calpain. Importantly, calpain inhibition is sufficient to preserve the molecular organization of the AIS both in vitro and in vivo. We conclude that loss of AIS ion channel clusters and neuronal polarity are important contributors to neuronal dysfunction after injury, and that strategies to facilitate recovery must preserve or repair the AIS cytoskeleton.
Figures
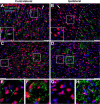
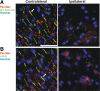
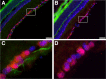
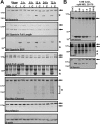


Similar articles
-
CaMKII phosphorylates collapsin response mediator protein 2 and modulates axonal damage during glutamate excitotoxicity.J Neurochem. 2009 Nov;111(3):870-81. doi: 10.1111/j.1471-4159.2009.06375.x. Epub 2009 Sep 7. J Neurochem. 2009. PMID: 19735446
-
Neurofascin assembles a specialized extracellular matrix at the axon initial segment.J Cell Biol. 2007 Aug 27;178(5):875-86. doi: 10.1083/jcb.200705119. Epub 2007 Aug 20. J Cell Biol. 2007. PMID: 17709431 Free PMC article.
-
αII Spectrin Forms a Periodic Cytoskeleton at the Axon Initial Segment and Is Required for Nervous System Function.J Neurosci. 2017 Nov 22;37(47):11311-11322. doi: 10.1523/JNEUROSCI.2112-17.2017. Epub 2017 Oct 16. J Neurosci. 2017. PMID: 29038240 Free PMC article.
-
Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon.Neurobiol Dis. 2013 Dec;60:61-79. doi: 10.1016/j.nbd.2013.08.010. Epub 2013 Aug 19. Neurobiol Dis. 2013. PMID: 23969238 Free PMC article. Review.
-
Functional implications of axon initial segment cytoskeletal disruption in stroke.Acta Pharmacol Sin. 2016 Jan;37(1):75-81. doi: 10.1038/aps.2015.107. Epub 2015 Dec 21. Acta Pharmacol Sin. 2016. PMID: 26687934 Free PMC article. Review.
Cited by
-
Alterations of the axon initial segment in multiple sclerosis grey matter.Brain Commun. 2022 Nov 4;4(6):fcac284. doi: 10.1093/braincomms/fcac284. eCollection 2022. Brain Commun. 2022. PMID: 36451656 Free PMC article.
-
Axon initial segment dysfunction in epilepsy.J Physiol. 2010 Jun 1;588(Pt 11):1829-40. doi: 10.1113/jphysiol.2010.188417. Epub 2010 Apr 7. J Physiol. 2010. PMID: 20375142 Free PMC article. Review.
-
Reduced axonal surface expression and phosphoinositide sensitivity in Kv7 channels disrupts their function to inhibit neuronal excitability in Kcnq2 epileptic encephalopathy.Neurobiol Dis. 2018 Oct;118:76-93. doi: 10.1016/j.nbd.2018.07.004. Epub 2018 Jul 6. Neurobiol Dis. 2018. PMID: 30008368 Free PMC article.
-
Localized Axolemma Deformations Suggest Mechanoporation as Axonal Injury Trigger.Front Neurol. 2020 Jan 30;11:25. doi: 10.3389/fneur.2020.00025. eCollection 2020. Front Neurol. 2020. PMID: 32082244 Free PMC article.
-
Handgrip-Related Activation in the Primary Motor Cortex Relates to Underlying Neuronal Metabolism After Stroke.Neurorehabil Neural Repair. 2014 Jun;28(5):433-42. doi: 10.1177/1545968313516868. Epub 2013 Dec 27. Neurorehabil Neural Repair. 2014. PMID: 24376066 Free PMC article.
References
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. - PubMed
-
- Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke. 2005;36:337–341. - PubMed
-
- Benn SC, Woolf CJ. Adult neuron survival strategies–slamming on the brakes. Nat Rev Neurosci. 2004;5:686–700. - PubMed
-
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS050505-05/NS/NINDS NIH HHS/United States
- R01 NS055215/NS/NINDS NIH HHS/United States
- T32 NS041224/NS/NINDS NIH HHS/United States
- 5T32NS041224/NS/NINDS NIH HHS/United States
- NS050505/NS/NINDS NIH HHS/United States
- NS055215/NS/NINDS NIH HHS/United States
- NS044916/NS/NINDS NIH HHS/United States
- R01 NS044916-09/NS/NINDS NIH HHS/United States
- R37 NS044916/NS/NINDS NIH HHS/United States
- R01 NS044916/NS/NINDS NIH HHS/United States
- R01 NS055215-04/NS/NINDS NIH HHS/United States
- R01 NS050505/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
