Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells
- PMID: 19846522
- PMCID: PMC2798399
- DOI: 10.1128/JVI.01690-09
Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells
Abstract
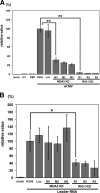
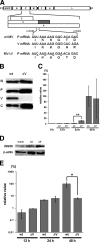
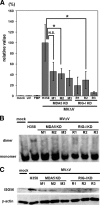
Measles virus (MV), a member of the family Paramyxoviridae, is a nonsegmented negative-strand RNA virus. The RNA helicases retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) are differentially involved in the detection of cytoplasmic viral RNAs and induction of alpha/beta interferon (IFN-alpha/beta). RIG-I is generally believed to play a major role in the recognition of paramyxoviruses, whereas many viruses of this family produce V proteins that can inhibit MDA5. To determine the individual roles of MDA5 and RIG-I in IFN induction after MV infection, small interfering RNA-mediated knockdown of MDA5 or RIG-I was performed in the human epithelial cell line H358, which is susceptible to wild-type MV isolates. The production of IFN-beta mRNA in response to MV infection was greatly reduced in RIG-I knockdown clones compared to that in H358 cells, confirming the importance of RIG-I in the detection of MV. The IFN-beta mRNA levels were also moderately reduced in MDA5 knockdown clones, even though these clones retained fully functional RIG-I. A V protein-deficient recombinant MV (MVDeltaV) induced higher amounts of IFN-beta mRNA at the early stage of infection in H358 cells compared to the parental virus. The reductions in the IFN-beta mRNA levels in RIG-I knockdown clones were less pronounced after infection with MVDeltaV than after infection with the parental virus. Taken together, the present results indicate that RIG-I and MDA5 both contribute to the recognition of MV and that the V protein promotes MV growth at least partly by inhibiting the MDA5-mediated IFN responses.
Figures


Similar articles
-
Strain-to-strain difference of V protein of measles virus affects MDA5-mediated IFN-β-inducing potential.Mol Immunol. 2011 Jan;48(4):497-504. doi: 10.1016/j.molimm.2010.10.006. Epub 2010 Nov 10. Mol Immunol. 2011. PMID: 21071089
-
MAVS-dependent IRF3/7 bypass of interferon β-induction restricts the response to measles infection in CD150Tg mouse bone marrow-derived dendritic cells.Mol Immunol. 2014 Feb;57(2):100-10. doi: 10.1016/j.molimm.2013.08.007. Epub 2013 Oct 4. Mol Immunol. 2014. PMID: 24096085
-
Sensing and control of bluetongue virus infection in epithelial cells via RIG-I and MDA5 helicases.J Virol. 2012 Nov;86(21):11789-99. doi: 10.1128/JVI.00430-12. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915805 Free PMC article.
-
Regulation of antiviral innate immune responses by RIG-I family of RNA helicases.Curr Top Microbiol Immunol. 2007;316:193-205. doi: 10.1007/978-3-540-71329-6_10. Curr Top Microbiol Immunol. 2007. PMID: 17969449 Review.
-
The regulation of type I interferon production by paramyxoviruses.J Interferon Cytokine Res. 2009 Sep;29(9):539-47. doi: 10.1089/jir.2009.0071. J Interferon Cytokine Res. 2009. PMID: 19702509 Free PMC article. Review.
Cited by
-
Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome.PLoS Pathog. 2012;8(8):e1002857. doi: 10.1371/journal.ppat.1002857. Epub 2012 Aug 9. PLoS Pathog. 2012. PMID: 22916014 Free PMC article.
-
Identification of human parainfluenza virus type 2 (HPIV-2) V protein amino acid residues that reduce binding of V to MDA5 and attenuate HPIV-2 replication in nonhuman primates.J Virol. 2011 Apr;85(8):4007-19. doi: 10.1128/JVI.02542-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289116 Free PMC article.
-
Measles virus C protein interferes with Beta interferon transcription in the nucleus.J Virol. 2012 Jan;86(2):796-805. doi: 10.1128/JVI.05899-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072748 Free PMC article.
-
Porcine circovirus type 2 upregulates endothelial-derived IL-8 production in porcine iliac artery endothelial cells via the RIG-I/MDA-5/MAVS/JNK signaling pathway.BMC Vet Res. 2020 Jul 29;16(1):265. doi: 10.1186/s12917-020-02486-1. BMC Vet Res. 2020. PMID: 32727484 Free PMC article.
-
Friend or foe: RIG- I like receptors and diseases.Autoimmun Rev. 2022 Oct;21(10):103161. doi: 10.1016/j.autrev.2022.103161. Epub 2022 Aug 1. Autoimmun Rev. 2022. PMID: 35926770 Free PMC article. Review.
References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

