Effects of 7,8-dihydro-8-oxo-deoxyguanosine on antigen challenge in ovalbumin-sensitized mice may be mediated by suppression of Rac
- PMID: 19845673
- PMCID: PMC2801215
- DOI: 10.1111/j.1476-5381.2009.00436.x
Effects of 7,8-dihydro-8-oxo-deoxyguanosine on antigen challenge in ovalbumin-sensitized mice may be mediated by suppression of Rac
Abstract
Background and purpose: Earlier we reported that 7,8-dihydro-8-oxo-deoxyguanosine (8-oxo-dG), an oxidatively modified guanine nucleoside, exerted anti-inflammatory activity through inactivation of the GTP binding protein, Rac. In the present study, the effects of 8-oxo-dG were investigated on responses to antigen challenge in sensitized mice, as Rac is also involved at several steps of the immune process including antigen-induced release of mediators from mast cells.
Experimental approach: Mice were sensitized and challenged with ovalbumin without or with oral administration of 8-oxo-dG during the challenge. Effects of 8-oxo-dG were assessed by measuring lung function, cells and cytokines in broncho-alveolar lavage fluid (BALF) and serum levels of antigen-specific IgE. Rac activity in BALF cells was also measured.
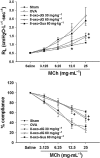
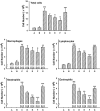
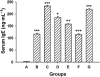
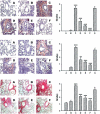
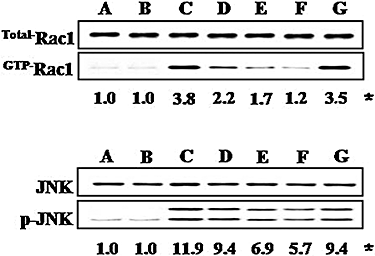
Key results: 8-oxo-dG inhibited the increased airway resistance and decreased lung compliance of sensitized and challenged mice to the levels of non-sensitized control mice and lowered the increased leukocytes particularly, eosinophils, in BALF. Furthermore, 8-oxo-dG suppressed allergy-associated immune responses, such as raised anti- ovalbumin IgE antibody in serum, increased expression of CD40 and CD40 ligand in lung, increased interleukin-4, -5, -13, interferon-gamma and tumour necrosis factor-alpha in BALF and mRNA levels of these cytokines in BALF cells, dose-dependently. The corresponding purine, 8-oxo-guanine, showed no effects in the same experiments. Finally, 8-oxo-dG, but not 8-oxo-guanine, inhibited the increased Rac activity in sensitized and challenged mice.
Conclusion and implications: 8-Oxo-dG had anti-allergic actions that might be mediated by Rac inactivation. This compound merits further evaluation of its therapeutic potential in allergic asthma.
Figures
Similar articles
-
8-oxo-2'-deoxyguanosine suppresses allergy-induced lung tissue remodeling in mice.Eur J Pharmacol. 2011 Jan 25;651(1-3):218-26. doi: 10.1016/j.ejphar.2010.10.087. Epub 2010 Nov 27. Eur J Pharmacol. 2011. PMID: 21114981
-
Expression of airway remodeling proteins in mast cell activated by TGF-β released in OVA-induced allergic responses and their inhibition by low-dose irradiation or 8-oxo-dG.Radiat Res. 2014 Apr;181(4):425-38. doi: 10.1667/RR13547.1. Epub 2014 Apr 10. Radiat Res. 2014. PMID: 24720751
-
[Combined effects of neonatal Bacillus Calmette-Guerin vaccination and respiratory syncytial infection on experimental asthma in mice].Zhonghua Er Ke Za Zhi. 2006 Jun;44(6):420-4. Zhonghua Er Ke Za Zhi. 2006. PMID: 16836848 Chinese.
-
The Influence of 2'-Deoxyguanosine Lesions on the Electronic Properties of OXOG:::C Base Pairs in Ds-DNA: A Comparative Analysis of Theoretical Studies.Molecules. 2024 Aug 8;29(16):3756. doi: 10.3390/molecules29163756. Molecules. 2024. PMID: 39202837 Free PMC article. Review.
-
Salvage of oxidized guanine derivatives in the (2'-deoxy)ribonucleotide pool as source of mutations in DNA.Mutat Res. 2010 Nov 28;703(1):11-7. doi: 10.1016/j.mrgentox.2010.08.021. Epub 2010 Sep 15. Mutat Res. 2010. PMID: 20833264 Free PMC article. Review.
Cited by
-
Addition of oh8dG to Cardioplegia Attenuated Myocardial Oxidative Injury through the Inhibition of Sodium Bicarbonate Cotransporter Activity.Antioxidants (Basel). 2022 Aug 24;11(9):1641. doi: 10.3390/antiox11091641. Antioxidants (Basel). 2022. PMID: 36139714 Free PMC article.
-
8-Hydroxy-2-deoxyguanosine prevents plaque formation and inhibits vascular smooth muscle cell activation through Rac1 inactivation.Free Radic Biol Med. 2012 Jul 1;53(1):109-21. doi: 10.1016/j.freeradbiomed.2012.03.023. Epub 2012 Apr 19. Free Radic Biol Med. 2012. PMID: 22580124 Free PMC article.
-
Investigating the Anti-Inflammatory Effects of RCI001 for Treating Ocular Surface Diseases: Insight Into the Mechanism of Action.Front Immunol. 2022 Mar 24;13:850287. doi: 10.3389/fimmu.2022.850287. eCollection 2022. Front Immunol. 2022. PMID: 35401555 Free PMC article.
-
8-Hydroxydeoxyguanosine: not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases.World J Gastroenterol. 2012 Jan 28;18(4):302-8. doi: 10.3748/wjg.v18.i4.302. World J Gastroenterol. 2012. PMID: 22294836 Free PMC article. Review.
-
Oxidized Cell-Free DNA Role in the Antioxidant Defense Mechanisms under Stress.Oxid Med Cell Longev. 2019 Jul 8;2019:1245749. doi: 10.1155/2019/1245749. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31360293 Free PMC article.
References
-
- Bromberg Y, Shani E, Joseph G, Gorzalczany Y, Sperling O, Pick E. The GDP-bound form of the small G protein Rac1 p21 is a potent activator of the superoxide-forming NADPH oxidase of macrophage. J Biol Chem. 1994;269:7055–7058. - PubMed
-
- Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, et al. Intranasal delivery of cytoplasmic domain of CTLA-4 using a novel protein transduction. Nat Med. 2006;12:574–579. - PubMed
-
- Choi S, Choi HH, Lee SH, Ko SH, You HJ, Ye SK, et al. Anti-inflammatory effects of 8-hydroxy-2′-deoxyguanosine on lipopolysaccharide-induced inflammation via Rac suppression in Balb/c mice. Free Rad Biol Med. 2007;43:1594–1603. - PubMed
-
- Gonzalez-Carmona MA, Lukacs-Kornek V, Timmerman A, Shabani S, Kornek M, Vogt A, et al. CD40ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology. 2008;48:157–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

