EBNA1-mediated recruitment of a histone H2B deubiquitylating complex to the Epstein-Barr virus latent origin of DNA replication
- PMID: 19834552
- PMCID: PMC2757719
- DOI: 10.1371/journal.ppat.1000624
EBNA1-mediated recruitment of a histone H2B deubiquitylating complex to the Epstein-Barr virus latent origin of DNA replication
Abstract
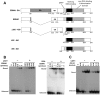
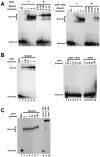
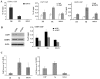
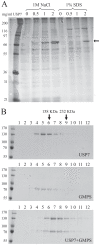
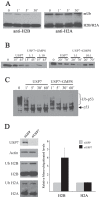
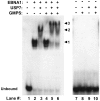
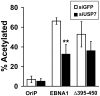
The EBNA1 protein of Epstein-Barr virus (EBV) plays essential roles in enabling the replication and persistence of EBV genomes in latently infected cells and activating EBV latent gene expression, in all cases by binding to specific recognition sites in the latent origin of replication, oriP. Here we show that EBNA1 binding to its recognition sites in vitro is greatly stimulated by binding to the cellular deubiquitylating enzyme, USP7, and that USP7 can form a ternary complex with DNA-bound EBNA1. Consistent with the in vitro effects, the assembly of EBNA1 on oriP elements in human cells was decreased by USP7 silencing, whereas assembly of an EBNA1 mutant defective in USP7 binding was unaffected. USP7 affinity column profiling identified a complex between USP7 and human GMP synthetase (GMPS), which was shown to stimulate the ability of USP7 to cleave monoubiquitin from histone H2B in vitro. Accordingly, silencing of USP7 in human cells resulted in a consistent increase in the level of monoubquitylated H2B. The USP7-GMPS complex formed a quaternary complex with DNA-bound EBNA1 in vitro and, in EBV infected cells, was preferentially detected at the oriP functional element, FR, along with EBNA1. Down-regulation of USP7 reduced the level of GMPS at the FR, increased the level of monoubiquitylated H2B in this region of the origin and decreased the ability of EBNA1, but not an EBNA1 USP7-binding mutant, to activate transcription from the FR. The results indicate that USP7 can stimulate EBNA1-DNA interactions and that EBNA1 can alter histone modification at oriP through recruitment of USP7.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
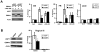
Similar articles
-
Structural Basis for Cooperative Binding of EBNA1 to the Epstein-Barr Virus Dyad Symmetry Minimal Origin of Replication.J Virol. 2019 Sep 30;93(20):e00487-19. doi: 10.1128/JVI.00487-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31142669 Free PMC article.
-
Structural and Functional Basis for an EBNA1 Hexameric Ring in Epstein-Barr Virus Episome Maintenance.J Virol. 2017 Sep 12;91(19):e01046-17. doi: 10.1128/JVI.01046-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701406 Free PMC article.
-
Cryo-EM Structure and Functional Studies of EBNA1 Binding to the Family of Repeats and Dyad Symmetry Elements of Epstein-Barr Virus oriP.J Virol. 2022 Sep 14;96(17):e0094922. doi: 10.1128/jvi.00949-22. Epub 2022 Aug 29. J Virol. 2022. PMID: 36037477 Free PMC article.
-
Replication licensing of the EBV oriP minichromosome.Curr Top Microbiol Immunol. 2001;258:13-33. doi: 10.1007/978-3-642-56515-1_2. Curr Top Microbiol Immunol. 2001. PMID: 11443858 Review.
-
EBNA1 and host factors in Epstein-Barr virus latent DNA replication.Curr Opin Virol. 2012 Dec;2(6):733-9. doi: 10.1016/j.coviro.2012.09.005. Epub 2012 Sep 30. Curr Opin Virol. 2012. PMID: 23031715 Review.
Cited by
-
Removal of H2Aub1 by ubiquitin-specific proteases 12 and 13 is required for stable Polycomb-mediated gene repression in Arabidopsis.Genome Biol. 2020 Jun 16;21(1):144. doi: 10.1186/s13059-020-02062-8. Genome Biol. 2020. PMID: 32546254 Free PMC article.
-
Epstein-Barr Virus Promotes B Cell Lymphomas by Manipulating the Host Epigenetic Machinery.Cancers (Basel). 2020 Oct 19;12(10):3037. doi: 10.3390/cancers12103037. Cancers (Basel). 2020. PMID: 33086505 Free PMC article. Review.
-
Transcription profiling of Epstein-Barr virus nuclear antigen (EBNA)-1 expressing cells suggests targeting of chromatin remodeling complexes.PLoS One. 2010 Aug 10;5(8):e12052. doi: 10.1371/journal.pone.0012052. PLoS One. 2010. PMID: 20706582 Free PMC article.
-
Human Herpesvirus 8 Interferon Regulatory Factors 1 and 3 Mediate Replication and Latency Activities via Interactions with USP7 Deubiquitinase.J Virol. 2018 Mar 14;92(7):e02003-17. doi: 10.1128/JVI.02003-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343584 Free PMC article.
-
Role of Virally-Encoded Deubiquitinating Enzymes in Regulation of the Virus Life Cycle.Int J Mol Sci. 2021 Apr 23;22(9):4438. doi: 10.3390/ijms22094438. Int J Mol Sci. 2021. PMID: 33922750 Free PMC article. Review.
References
-
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. fourth ed. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 2575–2627.
-
- Rawlins DR, Milman G, Hayward SD, Hayward GS. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA1) to clustered sites in the plasmid maintenance region. Cell. 1985;42:859–868. - PubMed
-
- Gahn TA, Schildkraut CL. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989;58:527–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

