Human regulatory T cells are targets for human immunodeficiency Virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain
- PMID: 19828616
- PMCID: PMC2786841
- DOI: 10.1128/JVI.01352-09
Human regulatory T cells are targets for human immunodeficiency Virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain
Abstract
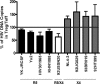
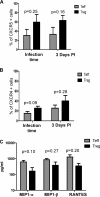
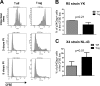
Regulatory T cells (Treg) are a subpopulation of CD4(+) T cells characterized by the suppressive activity they exert on effector immune responses, including human immunodeficiency virus (HIV)-specific immune responses. Because Treg express CXCR4 and CCR5, they represent potential targets for HIV; however, Treg susceptibility to HIV infection is still unclear. We therefore performed an extensive study of Treg susceptibility to HIV, using lab strains and primary isolates with either CCR5 or CXCR4 tropism. Furthermore, we quantified HIV infection at early and late time points of the virus life cycle. We found that Treg were clearly susceptible to HIV infection. Circulating Treg were not preferentially infected with HIV compared to effector T cells (Teff) in vivo. Conversely, in vitro infection with either CCR5-using (R5) or CXCR4-using (X4) viruses occurred with different dynamics. For instance, HIV infection by R5 viruses (lab strains and primary isolates) resulted in lower levels of infection in Treg compared with Teff at both early and late time points. In contrast, X4 viruses induced higher levels of infection in Treg compared to Teff at early time points, but this difference disappeared at the late time points of the virus life cycle. Our results suggest that the relative susceptibility of Treg to HIV infection compared to Teff varies, depending on both viral and host factors. These variations may play an important role in HIV pathogenesis.
Figures

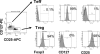


Similar articles
-
High CD4(+) T-cell surface CXCR4 density as a risk factor for R5 to X4 switch in the course of HIV-1 infection.J Acquir Immune Defic Syndr. 2010 Dec 15;55(5):529-35. doi: 10.1097/QAI.0b013e3181f25bab. J Acquir Immune Defic Syndr. 2010. PMID: 20861743
-
A CCR5+ memory subset within HIV-1-infected primary resting CD4+ T cells is permissive for replication-competent, latently infected viruses in vitro.BMC Res Notes. 2019 Apr 29;12(1):242. doi: 10.1186/s13104-019-4281-5. BMC Res Notes. 2019. PMID: 31036079 Free PMC article.
-
CCR5 and CXCR4 expression correlated with X4 and R5 HIV-1 infection yet not sustained replication in Th1 and Th2 cells.AIDS. 2001 Oct 19;15(15):1941-9. doi: 10.1097/00002030-200110190-00005. AIDS. 2001. PMID: 11600821
-
[Deep lung--cellular reaction to HIV].Rev Port Pneumol. 2007 Mar-Apr;13(2):175-212. Rev Port Pneumol. 2007. PMID: 17492233 Review. Portuguese.
-
Homeostasis and function of regulatory T cells in HIV/SIV infection.J Virol. 2012 Oct;86(19):10262-9. doi: 10.1128/JVI.00993-12. Epub 2012 Jul 18. J Virol. 2012. PMID: 22811537 Free PMC article. Review.
Cited by
-
Rapid and Slow Progressors Show Increased IL-6 and IL-10 Levels in the Pre-AIDS Stage of HIV Infection.PLoS One. 2016 May 23;11(5):e0156163. doi: 10.1371/journal.pone.0156163. eCollection 2016. PLoS One. 2016. PMID: 27214135 Free PMC article.
-
Schistosoma mansoni soluble egg antigen (SEA) and recombinant Omega-1 modulate induced CD4+ T-lymphocyte responses and HIV-1 infection in vitro.PLoS Pathog. 2019 Sep 5;15(9):e1007924. doi: 10.1371/journal.ppat.1007924. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31487324 Free PMC article.
-
Regulatory CD4 T cells inhibit HIV-1 expression of other CD4 T cell subsets via interactions with cell surface regulatory proteins.Virology. 2018 Mar;516:21-29. doi: 10.1016/j.virol.2017.12.036. Epub 2018 Jan 8. Virology. 2018. PMID: 29324358 Free PMC article.
-
Single-Cell Technologies Applied to HIV-1 Research: Reaching Maturity.Front Microbiol. 2020 Mar 4;11:297. doi: 10.3389/fmicb.2020.00297. eCollection 2020. Front Microbiol. 2020. PMID: 32194526 Free PMC article. Review.
-
Regulatory T Cells, Frailty, and Immune Activation in Men Who Have Sex With Men in the Multicenter AIDS Cohort Study.J Gerontol A Biol Sci Med Sci. 2015 Dec;70(12):1533-41. doi: 10.1093/gerona/glv132. Epub 2015 Aug 22. J Gerontol A Biol Sci Med Sci. 2015. PMID: 26297938 Free PMC article.
References
-
- Andersson, J., A. Boasso, J. Nilsson, R. Zhang, N. J. Shire, S. Lindback, G. M. Shearer, and C. A. Chougnet. 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143-3147. - PubMed
-
- Antons, A. K., R. Wang, K. Oswald-Richter, M. Tseng, C. W. Arendt, S. A. Kalams, and D. Unutmaz. 2008. Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J. Immunol. 180:764-773. - PubMed
-
- Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

