Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose
- PMID: 19817538
- PMCID: PMC2898921
- DOI: 10.3171/2009.9.JNS09844
Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose
Abstract
Object: This efficacy study was designed to investigate traumatic brain injury (TBI) in rats treated with delayed erythropoietin (EPO) administered in a single dose compared with a triple dose.
Methods: Young adult male Wistar rats were randomly divided into the following groups: 1) sham group (6 animals); 2) TBI/saline group (6 animals); 3) TBI/EPOx1 group (6 animals); and 4) TBI/EPOx3 group (7 animals). Traumatic brain injury was induced by controlled cortical impact over the left parietal cortex. Erythropoietin (5000 U/kg) or saline was administered intraperitoneally on Day 1 (EPOx1 group) or on Days 1, 2, and 3 (EPOx3 group) postinjury. Neurological function was assessed using a modified neurological severity score, foot-fault, and Morris water maze tests. Animals were killed 35 days after injury and brain sections were stained for immunohistochemistry.
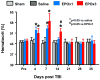
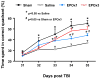
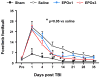
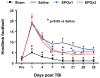
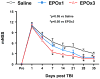
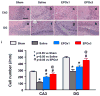
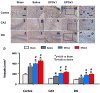
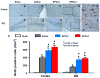
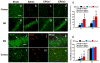
Results: Compared with the saline treatment, EPO treatment in both the EPOx1 and EPOx3 groups significantly reduced hippocampal cell loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, and significantly improved neurological functional outcome. The EPOx3 group exhibited significantly improved functional and histological outcomes compared with the EPOx1 group.
Conclusions: These data demonstrate that delayed posttraumatic administration of EPO significantly improved histological and long-term functional outcomes in rats after TBI. The triple doses of delayed EPO treatment produced better histological and functional outcomes in rats, although a single dose provided substantial benefits compared with saline treatment.
Figures
Similar articles
-
Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit.Brain Res. 2009 Oct 19;1294:153-64. doi: 10.1016/j.brainres.2009.07.077. Epub 2009 Jul 30. Brain Res. 2009. PMID: 19646970 Free PMC article.
-
Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury.J Neurosurg. 2011 Feb;114(2):549-59. doi: 10.3171/2010.10.JNS10925. Epub 2010 Nov 12. J Neurosurg. 2011. PMID: 21073254 Free PMC article.
-
Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats.J Neurosurg. 2011 Sep;115(3):550-60. doi: 10.3171/2011.3.JNS101721. Epub 2011 Apr 15. J Neurosurg. 2011. PMID: 21495821 Free PMC article.
-
The efficacy of erythropoietin in treating experimental traumatic brain injury: a systematic review of controlled trials in animal models.J Neurosurg. 2014 Sep;121(3):653-64. doi: 10.3171/2014.6.JNS132577. Epub 2014 Jul 18. J Neurosurg. 2014. PMID: 25036201 Review.
-
Angiogenesis, neurogenesis and brain recovery of function following injury.Curr Opin Investig Drugs. 2010 Mar;11(3):298-308. Curr Opin Investig Drugs. 2010. PMID: 20178043 Free PMC article. Review.
Cited by
-
Recombinant human erythropoietin increases cerebral cortical width index and neurogenesis following ischemic stroke.Neural Regen Res. 2012 Mar 15;7(8):578-82. doi: 10.3969/j.issn.1673-5374.2012.08.003. Neural Regen Res. 2012. PMID: 25745447 Free PMC article.
-
New horizons for newborn brain protection: enhancing endogenous neuroprotection.Arch Dis Child Fetal Neonatal Ed. 2015 Nov;100(6):F541-52. doi: 10.1136/archdischild-2014-306284. Epub 2015 Jun 10. Arch Dis Child Fetal Neonatal Ed. 2015. PMID: 26063194 Free PMC article. Review.
-
Reversal of established traumatic brain injury-induced, anxiety-like behavior in rats after delayed, post-injury neuroimmune suppression.J Neurotrauma. 2014 Mar 1;31(5):487-97. doi: 10.1089/neu.2013.3090. Epub 2013 Nov 20. J Neurotrauma. 2014. PMID: 24041015 Free PMC article.
-
Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma.Neurotherapeutics. 2014 Jul;11(3):679-95. doi: 10.1007/s13311-014-0277-y. Neurotherapeutics. 2014. PMID: 24965140 Free PMC article.
-
The Potential of Stem Cells in Treatment of Traumatic Brain Injury.Curr Neurol Neurosci Rep. 2018 Jan 25;18(1):1. doi: 10.1007/s11910-018-0812-z. Curr Neurol Neurosci Rep. 2018. PMID: 29372464 Free PMC article. Review.
References
-
- Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. - PubMed
-
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. - PubMed
-
- Cerami A. Beyond erythropoiesis: novel applications for recombinant human erythropoietin. Semin Hematol. 2001;38:33–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

