Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells
- PMID: 19815506
- PMCID: PMC2775313
- DOI: 10.1073/pnas.0905815106
Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells
Abstract
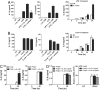
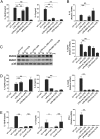
IL-10 produced by dendritic cells (DC) can limit or terminate ongoing inflammatory responses by inhibiting the proinflammatory cytokine production. Currently, the molecular mechanism by which IL-10 suppresses cytokine production is still ill-defined. In this study, we showed that IL-10 produced by DC dampens myeloid differentiation factor (MyD)88-dependent, but not MyD88-independent signaling. At the molecular level, IL-10 induces ubiquitination and subsequent protein degradation of MyD88-dependent signaling molecules, including IL-1 receptor-associated kinase 4 and TNF-receptor associated factor 6. Protein degradation by IL-10 was associated with decreased phosphorylation of p38, JNK, and IKK. All of these events were prevented by either blocking IL-10 receptor signaling or inhibiting proteasome degradation. IL-10 induced LPS hyporesponsiveness using the same mechanisms, i.e., ubiquitination and protein degradation. Thus, a previously undescribed regulatory mechanism by which IL-10-mediated protein degradation contributes to the inhibition of inflammatory cytokine production and endotoxin tolerance in DC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


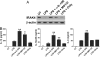
Similar articles
-
PKC-alpha controls MYD88-dependent TLR/IL-1R signaling and cytokine production in mouse and human dendritic cells.Eur J Immunol. 2010 Feb;40(2):505-15. doi: 10.1002/eji.200939391. Eur J Immunol. 2010. PMID: 19950169
-
Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling.J Leukoc Biol. 2009 Oct;86(4):863-75. doi: 10.1189/jlb.0309189. Epub 2009 Aug 5. J Leukoc Biol. 2009. PMID: 19656901 Free PMC article.
-
Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation.Int Immunol. 2002 Jul;14(7):695-700. doi: 10.1093/intimm/dxf039. Int Immunol. 2002. PMID: 12096028
-
LPS response and endotoxin tolerance in Flt-3L-induced bone marrow-derived dendritic cells.Cell Immunol. 2011;271(1):184-91. doi: 10.1016/j.cellimm.2011.06.020. Epub 2011 Jul 12. Cell Immunol. 2011. PMID: 21802073
-
Endotoxin-induced maturation of MyD88-deficient dendritic cells.J Immunol. 2001 May 1;166(9):5688-94. doi: 10.4049/jimmunol.166.9.5688. J Immunol. 2001. PMID: 11313410
Cited by
-
Divergent Roles of Kupffer Cell TLR2/3 Signaling in Alcoholic Liver Disease and the Protective Role of EGCG.Cell Mol Gastroenterol Hepatol. 2020;9(1):145-160. doi: 10.1016/j.jcmgh.2019.09.002. Epub 2019 Sep 25. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31562937 Free PMC article.
-
Myalgic encephalomyelitis or chronic fatigue syndrome: how could the illness develop?Metab Brain Dis. 2019 Apr;34(2):385-415. doi: 10.1007/s11011-019-0388-6. Epub 2019 Feb 13. Metab Brain Dis. 2019. PMID: 30758706 Free PMC article. Review.
-
TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity.Cancer Res. 2011 Aug 15;71(16):5467-76. doi: 10.1158/0008-5472.CAN-10-3988. Epub 2011 Jul 5. Cancer Res. 2011. PMID: 21730023 Free PMC article.
-
Daphnetin Alleviates Experimental Autoimmune Encephalomyelitis via Regulating Dendritic Cell Activity.CNS Neurosci Ther. 2016 Jul;22(7):558-67. doi: 10.1111/cns.12537. Epub 2016 Mar 25. CNS Neurosci Ther. 2016. PMID: 27013083 Free PMC article.
-
Dual Activation of TRIF and MyD88 Adaptor Proteins by Angiotensin II Evokes Opposing Effects on Pressure, Cardiac Hypertrophy, and Inflammatory Gene Expression.Hypertension. 2015 Sep;66(3):647-56. doi: 10.1161/HYPERTENSIONAHA.115.06011. Epub 2015 Jul 20. Hypertension. 2015. PMID: 26195481 Free PMC article.
References
-
- Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. - PubMed
-
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. - PubMed
-
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

