Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis
- PMID: 19805084
- PMCID: PMC2762680
- DOI: 10.1073/pnas.0909296106
Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis
Abstract
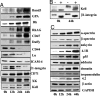
Erythropoiesis is the process by which nucleated erythroid progenitors proliferate and differentiate to generate, every second, millions of nonnucleated red cells with their unique discoid shape and membrane material properties. Here we examined the time course of appearance of individual membrane protein components during murine erythropoiesis to throw new light on our understanding of the evolution of the unique features of the red cell membrane. We found that the accumulation of all of the major transmembrane and all skeletal proteins of the mature red blood cell, except actin, accrued progressively during terminal erythroid differentiation. At the same time, and in marked contrast, accumulation of various adhesion molecules decreased. In particular, the adhesion molecule, CD44 exhibited a progressive and dramatic decrease from proerythroblast to reticulocyte; this enabled us to devise a new strategy for distinguishing unambiguously between erythroblasts at successive developmental stages. These findings provide unique insights into the genesis of red cell membrane function during erythroblast differentiation and also offer a means of defining stage-specific defects in erythroid maturation in inherited and acquired red cell disorders and in bone marrow failure syndromes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Membrane assembly during erythropoiesis.Curr Opin Hematol. 2011 May;18(3):133-8. doi: 10.1097/MOH.0b013e32834521f3. Curr Opin Hematol. 2011. PMID: 21372707 Review.
-
Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo.Blood. 2013 Apr 18;121(16):3246-53. doi: 10.1182/blood-2013-01-476390. Epub 2013 Feb 19. Blood. 2013. PMID: 23422750 Free PMC article.
-
Investigating the key membrane protein changes during in vitro erythropoiesis of protein 4.2 (-) cells (mutations Chartres 1 and 2).Haematologica. 2010 Aug;95(8):1278-86. doi: 10.3324/haematol.2009.021063. Epub 2010 Feb 23. Haematologica. 2010. PMID: 20179084 Free PMC article.
-
Erythroid differentiation denucleation factors (EDDFs) function as intrinsic, post-erythropoietin regulators for mammalian erythroid terminal differentiation.Cell Prolif. 2006 Feb;39(1):61-74. doi: 10.1111/j.1365-2184.2006.00366.x. Cell Prolif. 2006. PMID: 16426423 Free PMC article.
-
Novel methods for studying normal and disordered erythropoiesis.Sci China Life Sci. 2015 Dec;58(12):1270-5. doi: 10.1007/s11427-015-4971-8. Epub 2015 Nov 20. Sci China Life Sci. 2015. PMID: 26588913 Review.
Cited by
-
Erythroid Lineage Cells in the Liver: Novel Immune Regulators and Beyond.J Clin Transl Hepatol. 2020 Jun 28;8(2):177-183. doi: 10.14218/JCTH.2019.00031. Epub 2020 Jun 8. J Clin Transl Hepatol. 2020. PMID: 32832398 Free PMC article. Review.
-
Impairment of human terminal erythroid differentiation by histone deacetylase 5 deficiency.Blood. 2021 Oct 28;138(17):1615-1627. doi: 10.1182/blood.2020007401. Blood. 2021. PMID: 34036344 Free PMC article.
-
Molecular pathways of early CD105-positive erythroid cells as compared with CD34-positive common precursor cells by flow cytometric cell-sorting and gene expression profiling.Blood Cancer J. 2013 Jan 11;3(1):e100. doi: 10.1038/bcj.2012.45. Blood Cancer J. 2013. PMID: 23310930 Free PMC article.
-
Single-cell profiling of human megakaryocyte-erythroid progenitors identifies distinct megakaryocyte and erythroid differentiation pathways.Genome Biol. 2016 May 3;17:83. doi: 10.1186/s13059-016-0939-7. Genome Biol. 2016. PMID: 27142433 Free PMC article.
-
ABO gene editing for the conversion of blood type A to universal type O in Rhnull donor-derived human-induced pluripotent stem cells.Clin Transl Med. 2022 Oct;12(10):e1063. doi: 10.1002/ctm2.1063. Clin Transl Med. 2022. PMID: 36281739 Free PMC article.
References
-
- Gregory CJ, Eaves AC. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978;51:527–537. - PubMed
-
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989;988:107–121. - PubMed
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. - PubMed
-
- Discher DE. New insights into erythrocyte membrane organization and microelasticity. Curr Opin Hematol. 2000;7:117–122. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

