Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy
- PMID: 19794401
- PMCID: PMC2949111
- DOI: 10.1038/jcbfm.2009.197
Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy
Abstract
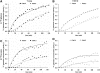
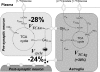
A decline in brain function is a characteristic feature of healthy aging; however, little is known about the biologic basis of this phenomenon. To determine whether there are alterations in brain mitochondrial metabolism associated with healthy aging, we combined (13)C/(1)H magnetic resonance spectroscopy with infusions of [1-(13)C]glucose and [2-(13)C]acetate to quantitatively characterize rates of neuronal and astroglial tricarboxylic acid cycles, as well as neuroglial glutamate-glutamine cycling, in healthy elderly and young volunteers. Compared with young subjects, neuronal mitochondrial metabolism and glutamate-glutamine cycle flux was approximately 30% lower in elderly subjects. The reduction in individual subjects correlated strongly with reductions in N-acetylaspartate and glutamate concentrations consistent with chronic reductions in brain mitochondrial function. In elderly subjects infused with [2-(13)C]acetate labeling of glutamine, C4 and C3 differed from that of the young subjects, indicating age-related changes in glial mitochondrial metabolism. Taken together, these studies show that healthy aging is associated with reduced neuronal mitochondrial metabolism and altered glial mitochondrial metabolism, which may in part be responsible for declines in brain function.
Figures


Similar articles
-
Metabolism of (1-(13)C) glucose and (2-(13)C, 2-(2)H(3)) acetate in the neuronal and glial compartments of the adult rat brain as detected by [(13)C, (2)H] NMR spectroscopy.Neurochem Int. 2000 Aug-Sep;37(2-3):217-28. doi: 10.1016/s0197-0186(00)00025-5. Neurochem Int. 2000. PMID: 10812207
-
The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression.Arch Gen Psychiatry. 2009 May;66(5):478-86. doi: 10.1001/archgenpsychiatry.2009.39. Arch Gen Psychiatry. 2009. PMID: 19414707
-
Aging alters the multichemical networking profile of the human brain: an in vivo (1)H-MRS study of young versus middle-aged subjects.J Neurochem. 2001 Apr;77(1):292-303. doi: 10.1046/j.1471-4159.2001.t01-1-00238.x. J Neurochem. 2001. PMID: 11279285 Clinical Trial.
-
Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings.Prog Neuropsychopharmacol Biol Psychiatry. 2006 Aug 30;30(6):969-95. doi: 10.1016/j.pnpbp.2006.03.012. Epub 2006 May 4. Prog Neuropsychopharmacol Biol Psychiatry. 2006. PMID: 16677749 Review.
-
Proton magnetic resonance spectroscopy of the human brain in schizophrenia.Rev Neurosci. 2000;11(2-3):147-58. doi: 10.1515/revneuro.2000.11.2-3.147. Rev Neurosci. 2000. PMID: 10718151 Review.
Cited by
-
In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2876-81. doi: 10.1073/pnas.1417921112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25730862 Free PMC article. Clinical Trial.
-
In vivo detection of 13C isotopomer turnover in the human brain by sequential infusion of 13C labeled substrates.J Magn Reson. 2012 May;218:16-21. doi: 10.1016/j.jmr.2012.03.012. Epub 2012 Mar 28. J Magn Reson. 2012. PMID: 22578550 Free PMC article.
-
Is there in vivo evidence for amino acid shuttles carrying ammonia from neurons to astrocytes?Neurochem Res. 2012 Nov;37(11):2597-612. doi: 10.1007/s11064-012-0898-7. Epub 2012 Oct 27. Neurochem Res. 2012. PMID: 23104556 Free PMC article. Review.
-
Local and Interregional Neurochemical Associations Measured by Magnetic Resonance Spectroscopy for Studying Brain Functions and Psychiatric Disorders.Front Psychiatry. 2020 Aug 11;11:802. doi: 10.3389/fpsyt.2020.00802. eCollection 2020. Front Psychiatry. 2020. PMID: 32848957 Free PMC article. Review.
-
Appraising the Role of Iron in Brain Aging and Cognition: Promises and Limitations of MRI Methods.Neuropsychol Rev. 2015 Sep;25(3):272-87. doi: 10.1007/s11065-015-9292-y. Epub 2015 Aug 7. Neuropsychol Rev. 2015. PMID: 26248580 Free PMC article. Review.
References
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Barker PB, Breiter SN, Soher BJ, Chatham JC, Forder JR, Samphilipo MA, Magee CA, Anderson JH. Quantitative proton spectroscopy of canine brain: in vivo and in vitro correlations. Magn Reson Med. 1994;32:157–163. - PubMed
-
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. - PubMed
-
- Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology. 2008;70:1353–1357. - PubMed
-
- Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, Pyne-Geithman GJ. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

