Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation
- PMID: 19786554
- PMCID: PMC5769454
- DOI: 10.4049/jimmunol.0900719
Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation
Abstract
IL-15 is a key component that regulates the development and homeostasis of NK cells and is delivered through a mechanism termed trans-presentation. During development, multiple events must proceed to generate a functional mature population of NK cells that are vital for tumor and viral immunity. Nevertheless, how IL-15 regulates these various events and more importantly what cells provide IL-15 to NK cells to drive these events is unclear. It is known dendritic cells (DC) can activate NK cells via IL-15 trans-presentation; however, the ability of DC to use IL-15 trans-presentation to promote the development and homeostatic maintenance of NK cell has not been established. In this current study, we show that IL-15 trans-presentation solely by CD11c(+) cells assists the in vivo development and maintenance of NK cells. More specifically, DC-mediated IL-15 trans-presentation drove the differentiation of NK cells, which included the up-regulation of the activating and inhibitory Ly49 receptors. Although these cells did not harbor a mature CD11b(high) phenotype, they were capable of degranulating and producing IFN-gamma upon stimulation similar to wild-type NK cells. In addition, DC facilitated the survival of mature NK cells via IL-15 trans-presentation in the periphery. Thus, an additional role for NK-DC interactions has been identified whereby DC support the developmental and homeostatic niche of NK cells.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
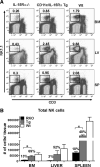
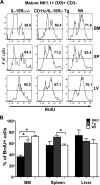
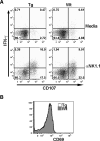
 ), and B6 (□) mice were calculated (average of three independent experiments, n = 7 mice/group). Error bars represent SE; *, p ≤ 0.05. Numbers above bars represent percentage of Wt levels.
), and B6 (□) mice were calculated (average of three independent experiments, n = 7 mice/group). Error bars represent SE; *, p ≤ 0.05. Numbers above bars represent percentage of Wt levels.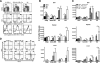
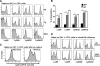
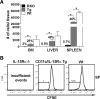
 ), and Wt (□) mice. Error bars represent SE, and the numbers above bars represent percentage of Wt levels; *, p ≤ 0.05. B, Dilution of CFSE by donor cells found in the spleen of the three hosts. Plots are representative of two individual experiments (n = 4 for each group).
), and Wt (□) mice. Error bars represent SE, and the numbers above bars represent percentage of Wt levels; *, p ≤ 0.05. B, Dilution of CFSE by donor cells found in the spleen of the three hosts. Plots are representative of two individual experiments (n = 4 for each group).Similar articles
-
Different NK cell developmental events require different levels of IL-15 trans-presentation.J Immunol. 2011 Aug 1;187(3):1212-21. doi: 10.4049/jimmunol.1100331. Epub 2011 Jun 29. J Immunol. 2011. PMID: 21715685
-
Transpresentation of interleukin-15 by IL-15/IL-15Rα mRNA-engineered human dendritic cells boosts antitumoral natural killer cell activity.Oncotarget. 2015 Dec 29;6(42):44123-33. doi: 10.18632/oncotarget.6536. Oncotarget. 2015. PMID: 26675759 Free PMC article.
-
NK cells provide helper signal for CD8+ T cells by inducing the expression of membrane-bound IL-15 on DCs.Int Immunol. 2009 May;21(5):599-606. doi: 10.1093/intimm/dxp029. Epub 2009 Mar 26. Int Immunol. 2009. PMID: 19325034
-
CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells.J Exp Med. 2007 Oct 29;204(11):2569-78. doi: 10.1084/jem.20071451. Epub 2007 Oct 8. J Exp Med. 2007. PMID: 17923507 Free PMC article.
-
Trans-presentation: a novel mechanism regulating IL-15 delivery and responses.Immunol Lett. 2010 Jan 4;127(2):85-92. doi: 10.1016/j.imlet.2009.09.009. Epub 2009 Oct 7. Immunol Lett. 2010. PMID: 19818367 Free PMC article. Review.
Cited by
-
M-CSF directs myeloid and NK cell differentiation to protect from CMV after hematopoietic cell transplantation.EMBO Mol Med. 2023 Nov 8;15(11):e17694. doi: 10.15252/emmm.202317694. Epub 2023 Aug 28. EMBO Mol Med. 2023. PMID: 37635627 Free PMC article.
-
IL-15 Superagonist-Mediated Immunotoxicity: Role of NK Cells and IFN-γ.J Immunol. 2015 Sep 1;195(5):2353-64. doi: 10.4049/jimmunol.1500300. Epub 2015 Jul 27. J Immunol. 2015. PMID: 26216888 Free PMC article.
-
IL-2 and Beyond in Cancer Immunotherapy.J Interferon Cytokine Res. 2018 Feb;38(2):45-68. doi: 10.1089/jir.2017.0101. J Interferon Cytokine Res. 2018. PMID: 29443657 Free PMC article. Review.
-
IL-15 Superagonist Expands mCD8+ T, NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection.PLoS One. 2016 Feb 9;11(2):e0148452. doi: 10.1371/journal.pone.0148452. eCollection 2016. PLoS One. 2016. PMID: 26859674 Free PMC article.
-
NK cell development, homeostasis and function: parallels with CD8⁺ T cells.Nat Rev Immunol. 2011 Aug 26;11(10):645-57. doi: 10.1038/nri3044. Nat Rev Immunol. 2011. PMID: 21869816 Free PMC article. Review.
References
-
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007;7:329–339. - PubMed
-
- Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 2002;3:523–528. - PubMed
-
- Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor γ-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 2005;174:1213–1221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

