Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells
- PMID: 19786546
- PMCID: PMC2788151
- DOI: 10.4049/jimmunol.0901531
Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells
Abstract
The CD200 receptor (CD200R) acts as a negative regulator of myeloid cells by interacting with its widely expressed ligand CD200. Using mutants expressed in U937 cells, we show that inhibition is mediated by the PTB domain binding motif (NPLY) in the receptor's cytoplasmic region. The adaptor protein downstream of tyrosine kinase 2 (Dok2) bound directly to the phosphorylated NPLY motif with a 10-fold higher affinity (K(D) of approximately 1 microM at 37 degrees C) than the closely related Dok1. Both of these proteins have been suggested to play a role in CD200R signaling in murine cells. Dok2 was phosphorylated in response to CD200R engagement and recruited RAS p21 protein activator 1 (RasGAP). Knockdown of Dok2 and RasGAP by RNA interference revealed that these proteins are required for CD200R signaling, while knockdown of Dok1 and the inositol 5-phosphatase SHIP did not affect CD200R-mediated inhibition. We conclude that CD200R inhibits the activation of human myeloid cells through direct recruitment of Dok2 and subsequent activation of RasGAP, which distinguishes this receptor from the majority of inhibitory receptors that utilize ITIMs and recruit phosphatases.
Figures
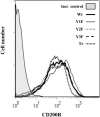
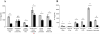
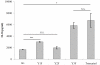


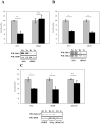
Similar articles
-
Downstream of tyrosine kinase 1 and 2 play opposing roles in CD200 receptor signaling.J Immunol. 2010 Dec 15;185(12):7216-22. doi: 10.4049/jimmunol.1002858. Epub 2010 Nov 15. J Immunol. 2010. PMID: 21078907 Free PMC article.
-
Molecular mechanisms of CD200 inhibition of mast cell activation.J Immunol. 2004 Dec 1;173(11):6786-93. doi: 10.4049/jimmunol.173.11.6786. J Immunol. 2004. PMID: 15557172
-
Identification of tyrosine residues crucial for CD200R-mediated inhibition of mast cell activation.J Leukoc Biol. 2006 Feb;79(2):363-8. doi: 10.1189/jlb.0705398. Epub 2005 Dec 5. J Leukoc Biol. 2006. PMID: 16330532
-
The roles of Dok family adapters in immunoreceptor signaling.Immunol Rev. 2009 Nov;232(1):273-85. doi: 10.1111/j.1600-065X.2009.00844.x. Immunol Rev. 2009. PMID: 19909370 Review.
-
p120-Ras GTPase activating protein (RasGAP): a multi-interacting protein in downstream signaling.Biochimie. 2009 Mar;91(3):320-8. doi: 10.1016/j.biochi.2008.10.010. Epub 2008 Oct 30. Biochimie. 2009. PMID: 19022332 Review.
Cited by
-
CD200 receptor restriction of myeloid cell responses antagonizes antiviral immunity and facilitates cytomegalovirus persistence within mucosal tissue.PLoS Pathog. 2015 Feb 5;11(2):e1004641. doi: 10.1371/journal.ppat.1004641. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25654642 Free PMC article.
-
Effects of Schistosoma haematobium infection and treatment on the systemic and mucosal immune phenotype, gene expression and microbiome: A systematic review.PLoS Negl Trop Dis. 2024 Sep 9;18(9):e0012456. doi: 10.1371/journal.pntd.0012456. eCollection 2024 Sep. PLoS Negl Trop Dis. 2024. PMID: 39250522 Free PMC article.
-
Quantitative Assessment of CD200 and CD200R Expression in Lung Cancer.Cancers (Basel). 2021 Mar 1;13(5):1024. doi: 10.3390/cancers13051024. Cancers (Basel). 2021. PMID: 33804482 Free PMC article.
-
Inhibition of CD200R1 expression by C/EBP β in reactive microglial cells.J Neuroinflammation. 2012 Jul 9;9:165. doi: 10.1186/1742-2094-9-165. J Neuroinflammation. 2012. PMID: 22776069 Free PMC article.
-
Porcine Macrophage Markers and Populations: An Update.Cells. 2023 Aug 19;12(16):2103. doi: 10.3390/cells12162103. Cells. 2023. PMID: 37626913 Free PMC article. Review.
References
-
- Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. - PubMed
-
- Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 2003;171:3034–3046. - PubMed
-
- Hatherley D, Barclay AN. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur. J. Immunol. 2004;34:1688–1694. - PubMed
-
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. - PubMed
-
- Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, Moshrefi MM, Gorman DM, Miller KL, Zhang S, Sedgwick JD, Phillips JH. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J. Immunol. 2005;174:1348–1356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

