Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities
- PMID: 19779023
- PMCID: PMC2773270
- DOI: 10.1093/hmg/ddp421
Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities
Abstract
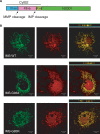
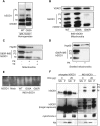
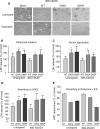
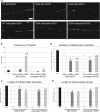
Amyotrophic lateral sclerosis (ALS) is a fatal neurological disorder characterized by motor neuron degeneration. Mutations in Cu,Zn-superoxide dismutase (SOD1) are responsible for 20% of familial ALS cases via a toxic gain of function. In mutant SOD1 transgenic mice, mitochondria of spinal motor neurons develop abnormal morphology, bioenergetic defects and degeneration, which are presumably implicated in disease pathogenesis. SOD1 is mostly a cytosolic protein, but a substantial portion is associated with organelles, including mitochondria, where it localizes predominantly in the intermembrane space (IMS). However, whether mitochondrial mutant SOD1 contributes to disease pathogenesis remains to be elucidated. We have generated NSC34 motor neuronal cell lines expressing wild-type or mutant SOD1 containing a cleavable IMS targeting signal to directly investigate the pathogenic role of mutant SOD1 in mitochondria. We show that mitochondrially-targeted SOD1 localizes to the IMS, where it is enzymatically active. We prove that mutant IMS-targeted SOD1 causes neuronal toxicity under metabolic and oxidative stress conditions. Furthermore, we demonstrate for the first time neurite mitochondrial fragmentation and impaired mitochondrial dynamics in motor neurons expressing IMS mutant SOD1. These defects are associated with impaired maintenance of neuritic processes. Our findings demonstrate that mutant SOD1 localized in the IMS is sufficient to determine mitochondrial abnormalities and neuronal toxicity, and contributes to ALS pathogenesis.
Figures

Similar articles
-
In vivo pathogenic role of mutant SOD1 localized in the mitochondrial intermembrane space.J Neurosci. 2011 Nov 2;31(44):15826-37. doi: 10.1523/JNEUROSCI.1965-11.2011. J Neurosci. 2011. PMID: 22049426 Free PMC article.
-
Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice.J Neurosci. 2005 Mar 9;25(10):2463-70. doi: 10.1523/JNEUROSCI.4385-04.2005. J Neurosci. 2005. PMID: 15758154 Free PMC article.
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space.Antioxid Redox Signal. 2010 Nov 1;13(9):1375-84. doi: 10.1089/ars.2010.3212. Antioxid Redox Signal. 2010. PMID: 20367259 Free PMC article. Review.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
Cited by
-
Significance of Programmed Cell Death Pathways in Neurodegenerative Diseases.Int J Mol Sci. 2024 Sep 15;25(18):9947. doi: 10.3390/ijms25189947. Int J Mol Sci. 2024. PMID: 39337436 Free PMC article. Review.
-
AAV-mediated upregulation of VDAC1 rescues the mitochondrial respiration and sirtuins expression in a SOD1 mouse model of inherited ALS.Cell Death Discov. 2024 Apr 16;10(1):178. doi: 10.1038/s41420-024-01949-w. Cell Death Discov. 2024. PMID: 38627359 Free PMC article.
-
Molecular hallmarks of ageing in amyotrophic lateral sclerosis.Cell Mol Life Sci. 2024 Mar 2;81(1):111. doi: 10.1007/s00018-024-05164-9. Cell Mol Life Sci. 2024. PMID: 38430277 Free PMC article. Review.
-
ERK1/2-dependent TSPO overactivation associates with the loss of mitophagy and mitochondrial respiration in ALS.Cell Death Dis. 2023 Feb 15;14(2):122. doi: 10.1038/s41419-023-05643-0. Cell Death Dis. 2023. PMID: 36792609 Free PMC article.
-
REEP1 Preserves Motor Function in SOD1G93A Mice by Improving Mitochondrial Function via Interaction with NDUFA4.Neurosci Bull. 2023 Jun;39(6):929-946. doi: 10.1007/s12264-022-00995-7. Epub 2022 Dec 15. Neurosci Bull. 2023. PMID: 36520405 Free PMC article.
References
-
- Bruijn L.I., Miller T.M., Cleveland D.W. Unraveling the mechanisms involved in motor neuron degeneration in Als. Annu. Rev. Neurosci. 2004;27:723–749. - PubMed
-
- Hervias I., Beal M.F., Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve. 2006;33:598–608. - PubMed
-
- Jung C., Higgins C.M., Xu Z. A quantitative histochemical assay for activities of mitochondrial electron transport chain complexes in mouse spinal cord sections. J. Neurosci. Methods. 2002;114:165–172. - PubMed
-
- Mattiazzi M., D'Aurelio M., Gajewski C.D., Martushova K., Kiaei M., Beal M.F., Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 2002;277:29626–29633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

