Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms
- PMID: 19768696
- PMCID: PMC2814307
- DOI: 10.1002/eji.200939441
Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms
Abstract
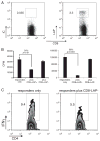
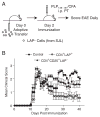
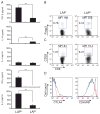
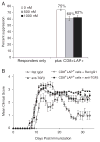
Although CD8+ Treg-mediated suppression has been described, CD8+ Treg remain poorly characterized. Here we identify a novel subset of CD8+ Treg that express latency-associated peptide (LAP) on their cell surface (CD8+LAP+ cells) and exhibit regulatory activity in vitro and in vivo. Only a small fraction of CD8+LAP+ cells express Foxp3 or CD25, although the expression levels of Foxp3 for these cells are higher than their LAP- counterparts. In addition to TGF-beta, CD8+LAP+ cells produce IFN-gamma, and these cells suppress EAE that is dependent on both TGF-beta and IFN-gamma. In an adoptive co-transfer model, CD8+LAP+ cells suppress myelin oligodendrocyte glycoprotein (MOG)-specific immune responses by inducing or expanding Foxp3+ cells and by inhibiting proliferation and IFN-gamma production in vivo. Furthermore, in vivo neutralization of IFN-gamma and studies with IFN-gamma-deficient mice demonstrate an important role for IFN-gamma production in the function of CD8+LAP+ cells. Our findings identify the underlying mechanisms that account for the immunoregulatory activity of CD8+ T cells and suggest that induction or amplification of CD8+LAP+ cells may be a therapeutic strategy to help control autoimmune processes.
Conflict of interest statement
Figures


Similar articles
-
Therapeutic role of interferon-γ in experimental autoimmune encephalomyelitis is mediated through a tolerogenic subset of splenic CD11b+ myeloid cells.J Neuroinflammation. 2024 May 31;21(1):144. doi: 10.1186/s12974-024-03126-3. J Neuroinflammation. 2024. PMID: 38822334 Free PMC article.
-
Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis.J Immunol. 2008 Jun 1;180(11):7327-37. doi: 10.4049/jimmunol.180.11.7327. J Immunol. 2008. PMID: 18490732 Free PMC article.
-
Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice.J Immunol. 2007 Jan 1;178(1):179-85. doi: 10.4049/jimmunol.178.1.179. J Immunol. 2007. PMID: 17182553
-
Dual roles of immune cells and their factors in cancer development and progression.Int J Biol Sci. 2011;7(5):651-8. doi: 10.7150/ijbs.7.651. Epub 2011 May 21. Int J Biol Sci. 2011. PMID: 21647333 Free PMC article. Review.
-
IFN-γ Contributes to the Immune Mechanisms of Hypertension.Kidney360. 2022 Oct 26;3(12):2164-2173. doi: 10.34067/KID.0001292022. eCollection 2022 Dec 29. Kidney360. 2022. PMID: 36591357 Free PMC article. Review.
Cited by
-
Functional characterization of HLA-G⁺ regulatory T cells in HIV-1 infection.PLoS Pathog. 2013 Jan;9(1):e1003140. doi: 10.1371/journal.ppat.1003140. Epub 2013 Jan 31. PLoS Pathog. 2013. PMID: 23382678 Free PMC article.
-
Immune-mediated processes in neurodegeneration: where do we stand?J Neurol. 2016 Sep;263(9):1683-701. doi: 10.1007/s00415-016-8052-0. Epub 2016 Feb 12. J Neurol. 2016. PMID: 26872669 Review.
-
Identification and characterization of latency-associated peptide-expressing γδ T cells.Nat Commun. 2015 Dec 8;6:8726. doi: 10.1038/ncomms9726. Nat Commun. 2015. PMID: 26644347 Free PMC article.
-
CD8+ T Lymphocytes: Crucial Players in Sjögren's Syndrome.Front Immunol. 2021 Jan 28;11:602823. doi: 10.3389/fimmu.2020.602823. eCollection 2020. Front Immunol. 2021. PMID: 33584670 Free PMC article. Review.
-
HLA-B7-restricted islet epitopes are differentially recognized in type 1 diabetic children and adults and form weak peptide-HLA complexes.Diabetes. 2012 Oct;61(10):2546-55. doi: 10.2337/db12-0136. Diabetes. 2012. PMID: 22997432 Free PMC article.
References
-
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. - PubMed
-
- Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. - PubMed
-
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. - PubMed
-
- Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. - PubMed
-
- Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

