Neuropathy target esterase is required for adult vertebrate axon maintenance
- PMID: 19759306
- PMCID: PMC3849655
- DOI: 10.1523/JNEUROSCI.3007-09.2009
Neuropathy target esterase is required for adult vertebrate axon maintenance
Abstract
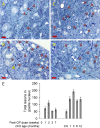
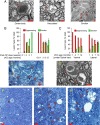
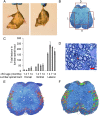
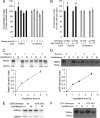
The enzyme neuropathy target esterase (NTE) is present in neurons and deacylates the major membrane phospholipid, phosphatidylcholine (PtdCho). Mutation of the NTE gene or poisoning by neuropathic organophosphates--chemical inhibitors of NTE--causes distal degeneration of long spinal axons in humans. However, analogous neuropathological changes have not been reported in nestin-cre:NTEfl/fl mice with NTE-deficient neural tissue. Furthermore, altered PtdCho homeostasis has not been detected in NTE-deficient vertebrates. Here, we describe distal degeneration of the longest spinal axons in approximately 3-week-old nestin-cre:NTEfl/fl mice and in adult C57BL/6J mice after acute dosing with a neuropathic organophosphate: in both groups early degenerative lesions were followed by swellings comprising accumulated axoplasmic material. In mice dosed acutely with organophosphate, maximal numbers of lesions, in the longest spinal sensory axon tract, were attained within days and were preceded by a transient rise in neural PtdCho. In nestin-cre:NTEfl/fl mice, sustained elevation of PtdCho over many months was accompanied by progressive degeneration and massive swelling of axons in sensory and motor spinal tracts and by increasing hindlimb dysfunction. Axonal lesion distribution closely resembled that in hereditary spastic paraplegia (HSP). The importance of defective membrane trafficking in HSP and the association of NTE with the endoplasmic reticulum--the starting point for the constitutive secretory pathway and transport of neuronal materials into axons--prompted investigation for a role of NTE in secretion. Cultured NTE-deficient neurons displayed modestly impaired secretion, consistent with neuronal viability and damage in vivo initially restricted to distal parts of the longest axons.
Figures
Similar articles
-
A mechanism for organophosphate-induced delayed neuropathy.Toxicol Lett. 2006 Mar 15;162(1):94-7. doi: 10.1016/j.toxlet.2005.10.012. Epub 2005 Nov 23. Toxicol Lett. 2006. PMID: 16309859
-
Organophosphates induce distal axonal damage, but not brain oedema, by inactivating neuropathy target esterase.Toxicol Appl Pharmacol. 2010 May 15;245(1):108-15. doi: 10.1016/j.taap.2010.02.010. Epub 2010 Feb 24. Toxicol Appl Pharmacol. 2010. PMID: 20188121
-
Neuronal phospholipid deacylation is essential for axonal and synaptic integrity.Biochim Biophys Acta. 2013 Mar;1831(3):633-41. doi: 10.1016/j.bbalip.2012.07.023. Epub 2012 Aug 7. Biochim Biophys Acta. 2013. PMID: 22903185 Review.
-
Axonal degeneration and neuropathy target esterase.Arh Hig Rada Toksikol. 2007 Sep;58(3):355-8. doi: 10.2478/v10004-007-0029-z. Arh Hig Rada Toksikol. 2007. PMID: 18050888 Review.
-
Neuropathy target esterase and phospholipid deacylation.Biochim Biophys Acta. 2005 Sep 15;1736(2):87-93. doi: 10.1016/j.bbalip.2005.08.002. Biochim Biophys Acta. 2005. PMID: 16137924 Review.
Cited by
-
A Study of Organophosphorus Induced Delayed Myelopathy: Uncommon Sequelae of a Common Poisoning.Ann Indian Acad Neurol. 2023 Jul-Aug;26(4):469-474. doi: 10.4103/aian.aian_19_23. Epub 2023 Apr 28. Ann Indian Acad Neurol. 2023. PMID: 37970320 Free PMC article.
-
Motor neuron disease due to neuropathy target esterase mutation: enzyme analysis of fibroblasts from human subjects yields insights into pathogenesis.Toxicol Lett. 2010 Nov 10;199(1):1-5. doi: 10.1016/j.toxlet.2010.06.020. Epub 2010 Sep 17. Toxicol Lett. 2010. PMID: 20603202 Free PMC article.
-
Organophosphate neurotoxicity to the voluntary motor system on the trail of environment-caused amyotrophic lateral sclerosis: the known, the misknown, and the unknown.Arch Toxicol. 2017 Aug;91(8):2939-2952. doi: 10.1007/s00204-016-1926-1. Epub 2017 Jan 9. Arch Toxicol. 2017. PMID: 28070599 Free PMC article. Review.
-
Disturbed phospholipid homeostasis in endoplasmic reticulum initiates tri-o-cresyl phosphate-induced delayed neurotoxicity.Sci Rep. 2016 Nov 24;6:37574. doi: 10.1038/srep37574. Sci Rep. 2016. PMID: 27883027 Free PMC article.
-
Functional pathways altered after silencing Pnpla6 (the codifying gene of neuropathy target esterase) in mouse embryonic stem cells under differentiation.In Vitro Cell Dev Biol Anim. 2014 Mar;50(3):261-73. doi: 10.1007/s11626-013-9691-4. Epub 2013 Oct 19. In Vitro Cell Dev Biol Anim. 2014. PMID: 24142151
References
-
- Cavanagh JB. The significance of the “dying-back” process in experimental and human neurological disease. Int Rev Exp Pathol. 1964;3:219–267. - PubMed
-
- Deluca GC, Ebers GC, Esiri MM. The extent of axonal loss in the long tracts in hereditary spastic paraplegia. Neuropathol Appl Neurobiol. 2004;30:576–584. - PubMed
-
- Glynn P. Neural development and neurodegeneration: two faces of neuropathy target esterase. Prog Neurobiol. 2000;61:61–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
