Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation
- PMID: 19755709
- PMCID: PMC2810640
- DOI: 10.1126/scisignal.2000368
Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation
Abstract
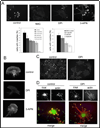
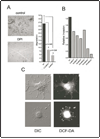
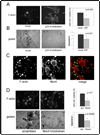
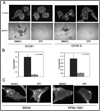
Invadopodia are actin-rich membrane protrusions of cancer cells that facilitate pericellular proteolysis and invasive behavior. We show here that reactive oxygen species (ROS) generated by the NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase (Nox) system are necessary for invadopodia formation and function. Knockdown of the invadopodia protein Tks5 [tyrosine kinase substrate with five Src homology 3 (SH3) domains], which is structurally related to the Nox component p47(phox), reduces total ROS abundance in cancer cells. Furthermore, Tks5 and p22(phox) can associate with each other, suggesting that Tks5 is part of the Nox complex. Tyrosine phosphorylation of Tks5 and Tks4, but not other Src substrates, is reduced by Nox inhibition. We propose that Tks5 facilitates the production of ROS necessary for invadopodia formation, and that in turn ROS modulate Tks5 tyrosine phosphorylation in a positive feedback loop.
Figures
Similar articles
-
Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity.Sci Signal. 2009 Sep 15;2(88):ra54. doi: 10.1126/scisignal.2000370. Sci Signal. 2009. PMID: 19755710 Free PMC article.
-
c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells.Mol Biol Cell. 2010 Dec;21(23):4287-98. doi: 10.1091/mbc.E10-08-0685. Epub 2010 Oct 13. Mol Biol Cell. 2010. PMID: 20943948 Free PMC article.
-
A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo.Eur J Cell Biol. 2008 Sep;87(8-9):555-67. doi: 10.1016/j.ejcb.2008.02.008. Epub 2008 Apr 15. Eur J Cell Biol. 2008. PMID: 18417249 Free PMC article.
-
NADPH OXIDASE: STRUCTURE AND ACTIVATION MECHANISMS (REVIEW). NOTE I.Rev Med Chir Soc Med Nat Iasi. 2016 Jan-Mar;120(1):29-33. Rev Med Chir Soc Med Nat Iasi. 2016. PMID: 27125069 Review.
-
Tks adaptor proteins at a glance.J Cell Sci. 2018 Jan 8;131(1):jcs203661. doi: 10.1242/jcs.203661. J Cell Sci. 2018. PMID: 29311151 Free PMC article. Review.
Cited by
-
Nox4 and diabetic nephropathy: with a friend like this, who needs enemies?Free Radic Biol Med. 2013 Aug;61:130-42. doi: 10.1016/j.freeradbiomed.2013.03.014. Epub 2013 Mar 23. Free Radic Biol Med. 2013. PMID: 23528476 Free PMC article. Review.
-
Signaling inputs to invadopodia and podosomes.J Cell Sci. 2013 Jul 15;126(Pt 14):2979-89. doi: 10.1242/jcs.079475. Epub 2013 Jul 10. J Cell Sci. 2013. PMID: 23843616 Free PMC article. Review.
-
Aiding and abetting roles of NOX oxidases in cellular transformation.Nat Rev Cancer. 2012 Sep;12(9):627-37. doi: 10.1038/nrc3339. Nat Rev Cancer. 2012. PMID: 22918415 Free PMC article. Review.
-
NADPH Oxidases NOXs and DUOXs as putative targets for cancer therapy.Anticancer Agents Med Chem. 2013 Mar;13(3):502-14. Anticancer Agents Med Chem. 2013. PMID: 22931418 Free PMC article. Review.
-
Invadopodia-mediated ECM degradation is enhanced in the G1 phase of the cell cycle.J Cell Sci. 2019 Oct 18;132(20):jcs227116. doi: 10.1242/jcs.227116. J Cell Sci. 2019. PMID: 31533971 Free PMC article.
References
-
- Linder S, Kopp P. Podosomes at a glance. J Cell Sci. 2005;118:2079. - PubMed
-
- Evans JG, Matsudaira P. Structure and dynamics of macrophage podosomes. Eur J Cell Biol. 2006;85:145. - PubMed
-
- Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97. - PubMed
-
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107. - PubMed
-
- Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

