Chapter 4: Multitasking by exploitation of intracellular transport functions the many faces of FcRn
- PMID: 19755184
- PMCID: PMC4485553
- DOI: 10.1016/S0065-2776(09)03004-1
Chapter 4: Multitasking by exploitation of intracellular transport functions the many faces of FcRn
Abstract
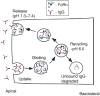
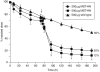
The MHC Class I-related receptor, FcRn, transports antibodies of the immunoglobulin G (IgG) class within and across a diverse array of different cell types. Through this transport, FcRn serves multiple roles throughout adult life that extend well beyond its earlier defined function of transcytosing IgGs from mother to offspring. These roles include the maintenance of IgG levels and the delivery of antigen in the form of immune complexes to degradative compartments within cells. Recent studies have led to significant advances in knowledge of the intracellular trafficking of FcRn and (engineered) IgGs at both the molecular and cellular levels. The engineering of FcRn-IgG (or Fc) interactions to generate antibodies of increased longevity represents an area of active interest, particularly in the light of the expanding use of antibodies in therapy. The strict pH dependence of FcRn-IgG interactions, with binding at pH 6 that becomes essentially undetectable as near neutral pH is approached, is essential for efficient transport. The requirement for retention of low affinity at near neutral pH increases the complexity of engineering antibodies for increased half-life. Conversely, engineered IgGs that have gained significant binding for FcRn at this pH can be potent inhibitors of FcRn that lower endogenous IgG levels and have multiple potential uses as therapeutics. In addition, molecular studies of FcRn-IgG interactions indicate that mice have limitations as preclinical models for FcRn function, primarily due to cross-species differences in FcRn-binding specificity.
Figures
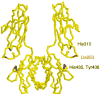



Similar articles
-
Targeting FcRn for the modulation of antibody dynamics.Mol Immunol. 2015 Oct;67(2 Pt A):131-41. doi: 10.1016/j.molimm.2015.02.007. Epub 2015 Mar 9. Mol Immunol. 2015. PMID: 25766596 Free PMC article. Review.
-
Multiple roles for the major histocompatibility complex class I- related receptor FcRn.Annu Rev Immunol. 2000;18:739-66. doi: 10.1146/annurev.immunol.18.1.739. Annu Rev Immunol. 2000. PMID: 10837074 Review.
-
Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels.Nat Biotechnol. 2005 Oct;23(10):1283-8. doi: 10.1038/nbt1143. Epub 2005 Sep 25. Nat Biotechnol. 2005. PMID: 16186811
-
Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments.J Immunol. 2008 Dec 1;181(11):7550-61. doi: 10.4049/jimmunol.181.11.7550. J Immunol. 2008. PMID: 19017944 Free PMC article.
-
Qualification of a homogeneous cell-based neonatal Fc receptor (FcRn) binding assay and its application to studies on Fc functionality of IgG-based therapeutics.J Immunol Methods. 2013 Apr 30;390(1-2):81-91. doi: 10.1016/j.jim.2013.01.011. Epub 2013 Feb 4. J Immunol Methods. 2013. PMID: 23384837
Cited by
-
Using multifocal plane microscopy to reveal novel trafficking processes in the recycling pathway.J Cell Sci. 2013 Mar 1;126(Pt 5):1176-88. doi: 10.1242/jcs.116327. Epub 2013 Jan 23. J Cell Sci. 2013. PMID: 23345403 Free PMC article.
-
Clinical chemistry of human FcRn transgenic mice.Mamm Genome. 2012 Apr;23(3-4):259-69. doi: 10.1007/s00335-011-9379-6. Epub 2011 Dec 23. Mamm Genome. 2012. PMID: 22193411
-
Recent advances using FcRn overexpression in transgenic animals to overcome impediments of standard antibody technologies to improve the generation of specific antibodies.MAbs. 2011 Sep-Oct;3(5):431-9. doi: 10.4161/mabs.3.5.17023. Epub 2011 Sep 1. MAbs. 2011. PMID: 22048692 Free PMC article. Review.
-
Half-life-extended recombinant coagulation factor IX-albumin fusion protein is recycled via the FcRn-mediated pathway.J Biol Chem. 2018 Apr 27;293(17):6363-6373. doi: 10.1074/jbc.M117.817064. Epub 2018 Mar 9. J Biol Chem. 2018. PMID: 29523681 Free PMC article.
-
Engineered clearing agents for the selective depletion of antigen-specific antibodies.Nat Commun. 2017 May 31;8:15314. doi: 10.1038/ncomms15314. Nat Commun. 2017. PMID: 28561044 Free PMC article.
References
-
- Adamski FM, King AT, Demmer J. Expression of the Fc receptor in the mammary gland during lactation in the marsupial Trichosurus vulpecula (brushtail possum) Mol Immunol. 2000;37:435–444. - PubMed
-
- Ahouse JJ, Hagerman CL, Mittal P, Gilbert DJ, Copeland NG, Jenkins NA, Simister NE. Mouse MHC class I-like Fc receptor encoded outside the MHC. J Immunol. 1993;151:6076–6088. - PubMed
-
- Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–4588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

