Promiscuous substrate recognition in folding and assembly activities of the trigger factor chaperone
- PMID: 19737520
- PMCID: PMC2799252
- DOI: 10.1016/j.cell.2009.07.044
Promiscuous substrate recognition in folding and assembly activities of the trigger factor chaperone
Abstract
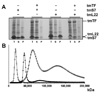
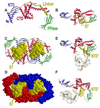
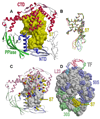
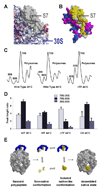
Trigger factor (TF) is a molecular chaperone that binds to bacterial ribosomes where it contacts emerging nascent chains, but TF is also abundant free in the cytosol where its activity is less well characterized. In vitro studies show that TF promotes protein refolding. We find here that ribosome-free TF stably associates with and rescues from misfolding a large repertoire of full-length proteins. We identify over 170 members of this cytosolic Escherichia coli TF substrate proteome, including ribosomal protein S7. We analyzed the biochemical properties of a TF:S7 complex from Thermotoga maritima and determined its crystal structure. Thereby, we obtained an atomic-level picture of a promiscuous chaperone in complex with a physiological substrate protein. The structure of the complex reveals the molecular basis of substrate recognition by TF, indicates how TF could accelerate protein folding, and suggests a role for TF in the biogenesis of protein complexes.
Figures


Similar articles
-
Structures of and interactions between domains of trigger factor from Thermotoga maritima.Acta Crystallogr D Biol Crystallogr. 2007 Apr;63(Pt 4):536-47. doi: 10.1107/S090744490700964X. Epub 2007 Mar 16. Acta Crystallogr D Biol Crystallogr. 2007. PMID: 17372359
-
Functional adaptations of the bacterial chaperone trigger factor to extreme environmental temperatures.Environ Microbiol. 2015 Jul;17(7):2407-20. doi: 10.1111/1462-2920.12707. Epub 2015 Jan 27. Environ Microbiol. 2015. PMID: 25389111
-
Versatility of trigger factor interactions with ribosome-nascent chain complexes.J Biol Chem. 2010 Sep 3;285(36):27911-23. doi: 10.1074/jbc.M110.134163. Epub 2010 Jul 1. J Biol Chem. 2010. PMID: 20595383 Free PMC article.
-
A cradle for new proteins: trigger factor at the ribosome.Curr Opin Struct Biol. 2005 Apr;15(2):204-12. doi: 10.1016/j.sbi.2005.03.005. Curr Opin Struct Biol. 2005. PMID: 15837180 Review.
-
Structure and function of the molecular chaperone Trigger Factor.Biochim Biophys Acta. 2010 Jun;1803(6):650-61. doi: 10.1016/j.bbamcr.2010.01.017. Epub 2010 Feb 2. Biochim Biophys Acta. 2010. PMID: 20132842 Review.
Cited by
-
How Quality Control Systems AID Sec-Dependent Protein Translocation.Front Mol Biosci. 2021 Apr 13;8:669376. doi: 10.3389/fmolb.2021.669376. eCollection 2021. Front Mol Biosci. 2021. PMID: 33928127 Free PMC article. Review.
-
Protein folding while chaperone bound is dependent on weak interactions.Nat Commun. 2019 Oct 23;10(1):4833. doi: 10.1038/s41467-019-12774-6. Nat Commun. 2019. PMID: 31645566 Free PMC article.
-
Structural and Kinetic Views of Molecular Chaperones in Multidomain Protein Folding.Int J Mol Sci. 2022 Feb 24;23(5):2485. doi: 10.3390/ijms23052485. Int J Mol Sci. 2022. PMID: 35269628 Free PMC article. Review.
-
Selective ribosome profiling as a tool for studying the interaction of chaperones and targeting factors with nascent polypeptide chains and ribosomes.Nat Protoc. 2013 Nov;8(11):2212-39. doi: 10.1038/nprot.2013.133. Epub 2013 Oct 17. Nat Protoc. 2013. PMID: 24136347 Free PMC article.
-
Reshaping of the conformational search of a protein by the chaperone trigger factor.Nature. 2013 Aug 1;500(7460):98-101. doi: 10.1038/nature12293. Epub 2013 Jul 7. Nature. 2013. PMID: 23831649
References
-
- Agashe VR, Guha S, Chang HC, Genevaux P, Hayer-Hartl M, Stemp M, Georgopoulos C, Hartl FU, Barral JM. Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell. 2004;117:199–209. - PubMed
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. - PubMed
-
- Brodersen DE, Clemons WM, Jr, Carter AP, Wimberly BT, Ramakrishnan V. Crystal structure of the 30 S ribosomal subunit from Thermus thermophilus: structure of the proteins and their interactions with 16 S RNA. J. Mol. Biol. 2002;316:725–768. - PubMed
-
- Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, Canadien V, Starostine A, Richards D, Beattie B, Krogan N, et al. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature. 2005;433:531–537. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

