HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation
- PMID: 19730691
- PMCID: PMC2729927
- DOI: 10.1371/journal.ppat.1000574
HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation
Abstract
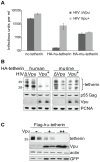
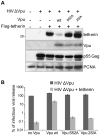
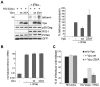
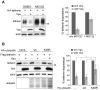
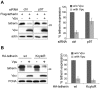
Host cells impose a broad range of obstacles to the replication of retroviruses. Tetherin (also known as CD317, BST-2 or HM1.24) impedes viral release by retaining newly budded HIV-1 virions on the surface of cells. HIV-1 Vpu efficiently counteracts this restriction. Here, we show that HIV-1 Vpu induces the depletion of tetherin from cells. We demonstrate that this phenomenon correlates with the ability of Vpu to counteract the antiviral activity of both overexpressed and interferon-induced endogenous tetherin. In addition, we show that Vpu co-immunoprecipitates with tetherin and beta-TrCP in a tri-molecular complex. This interaction leads to Vpu-mediated proteasomal degradation of tetherin in a beta-TrCP2-dependent manner. Accordingly, in conditions where Vpu-beta-TrCP2-tetherin interplay was not operative, including cells stably knocked down for beta-TrCP2 expression or cells expressing a dominant negative form of beta-TrCP, the ability of Vpu to antagonize the antiviral activity of tetherin was severely impaired. Nevertheless, tetherin degradation did not account for the totality of Vpu-mediated counteraction against the antiviral factor, as binding of Vpu to tetherin was sufficient for a partial relief of the restriction. Finally, we show that the mechanism used by Vpu to induce tetherin depletion implicates the cellular ER-associated degradation (ERAD) pathway, which mediates the dislocation of ER membrane proteins into the cytosol for subsequent proteasomal degradation. In conclusion, we show that Vpu interacts with tetherin to direct its beta-TrCP2-dependent proteasomal degradation, thereby alleviating the blockade to the release of infectious virions. Identification of tetherin binding to Vpu provides a potential novel target for the development of drugs aimed at inhibiting HIV-1 replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


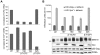

Similar articles
-
β-TrCP is dispensable for Vpu's ability to overcome the CD317/Tetherin-imposed restriction to HIV-1 release.Retrovirology. 2011 Feb 10;8:9. doi: 10.1186/1742-4690-8-9. Retrovirology. 2011. PMID: 21310048 Free PMC article.
-
Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment.PLoS Pathog. 2010 Apr 8;6(4):e1000856. doi: 10.1371/journal.ppat.1000856. PLoS Pathog. 2010. PMID: 20386718 Free PMC article.
-
Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism.J Virol. 2009 Aug;83(16):7931-47. doi: 10.1128/JVI.00242-09. Epub 2009 Jun 10. J Virol. 2009. PMID: 19515779 Free PMC article.
-
β-TrCP dependency of HIV-1 Vpu-induced downregulation of CD4 and BST-2/tetherin.Curr HIV Res. 2012 Jun;10(4):307-14. doi: 10.2174/157016212800792441. Curr HIV Res. 2012. PMID: 22524179 Review.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
Cited by
-
Breaking Barriers to an AIDS Model with Macaque-Tropic HIV-1 Derivatives.Biology (Basel). 2012 May 12;1(2):134-64. doi: 10.3390/biology1020134. Biology (Basel). 2012. PMID: 23336082 Free PMC article.
-
Mechanism of HIV-1 virion entrapment by tetherin.PLoS Pathog. 2013;9(7):e1003483. doi: 10.1371/journal.ppat.1003483. Epub 2013 Jul 18. PLoS Pathog. 2013. PMID: 23874200 Free PMC article.
-
Restriction of Retroviral Replication by Tetherin/BST-2.Mol Biol Int. 2012;2012:424768. doi: 10.1155/2012/424768. Epub 2012 Jul 2. Mol Biol Int. 2012. PMID: 22811908 Free PMC article.
-
The Vpu protein: new concepts in virus release and CD4 down-modulation.Curr HIV Res. 2010 Apr;8(3):240-52. doi: 10.2174/157016210791111124. Curr HIV Res. 2010. PMID: 20201792 Free PMC article. Review.
-
SIV Nef proteins recruit the AP-2 complex to antagonize Tetherin and facilitate virion release.PLoS Pathog. 2011 May;7(5):e1002039. doi: 10.1371/journal.ppat.1002039. Epub 2011 May 19. PLoS Pathog. 2011. PMID: 21625568 Free PMC article.
References
-
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. - PubMed
-
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, et al. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

