G-protein-coupled receptor oligomers: two or more for what? Lessons from mGlu and GABAB receptors
- PMID: 19723778
- PMCID: PMC2793868
- DOI: 10.1113/jphysiol.2009.179978
G-protein-coupled receptor oligomers: two or more for what? Lessons from mGlu and GABAB receptors
Abstract
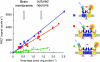
G-protein-coupled receptors (GPCRs) are key players in the precise tuning of intercellullar communication. In the brain, both major neurotransmitters, glutamate and GABA, act on specific GPCRs [the metabotropic glutamate (mGlu) and GABA(B) receptors] to modulate synaptic transmission. These receptors are encoded by the largest gene family, and have been found to associate into both homo- and hetero-oligomers, which increases the complexity of this cell communication system. Here we show that dimerization is required for mGlu and GABA(B) receptors to function, since the activation process requires a relative movement between the subunits to occur. We will also show that, in contrast to the mGlu receptors, which form strict dimers, the GABA(B) receptors assemble into larger complexes, both in transfected cells and in the brain, resulting in a decreased G-protein coupling efficacy. We propose that GABA(B) receptor oligomerization offers a way to increase the possibility of modulating receptor signalling and activity, allowing the same receptor protein to have specific properties in neurons at different locations.
Figures
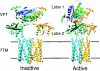


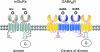

Similar articles
-
Asymmetric activation of dimeric GABAB and metabotropic glutamate receptors.Am J Physiol Cell Physiol. 2023 Jul 1;325(1):C79-C89. doi: 10.1152/ajpcell.00150.2022. Epub 2023 May 15. Am J Physiol Cell Physiol. 2023. PMID: 37184233 Review.
-
Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration.EMBO J. 2009 Aug 5;28(15):2195-208. doi: 10.1038/emboj.2009.177. Epub 2009 Jul 9. EMBO J. 2009. PMID: 19590495 Free PMC article.
-
Organization and functions of mGlu and GABAB receptor complexes.Nature. 2016 Dec 1;540(7631):60-68. doi: 10.1038/nature20566. Nature. 2016. PMID: 27905440 Review.
-
KCTD Hetero-oligomers Confer Unique Kinetic Properties on Hippocampal GABAB Receptor-Induced K+ Currents.J Neurosci. 2017 Feb 1;37(5):1162-1175. doi: 10.1523/JNEUROSCI.2181-16.2016. Epub 2016 Dec 21. J Neurosci. 2017. PMID: 28003345 Free PMC article.
-
The complexity of their activation mechanism opens new possibilities for the modulation of mGlu and GABAB class C G protein-coupled receptors.Neuropharmacology. 2011 Jan;60(1):82-92. doi: 10.1016/j.neuropharm.2010.08.009. Epub 2010 Aug 14. Neuropharmacology. 2011. PMID: 20713070 Review.
Cited by
-
Transmembrane domains of attraction on the TSH receptor.Endocrinology. 2015 Feb;156(2):488-98. doi: 10.1210/en.2014-1509. Epub 2014 Nov 19. Endocrinology. 2015. PMID: 25406938 Free PMC article.
-
Spinal Inhibition of GABAB Receptors by the Extracellular Matrix Protein Fibulin-2 in Neuropathic Rats.Front Cell Neurosci. 2020 Jul 15;14:214. doi: 10.3389/fncel.2020.00214. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32765223 Free PMC article.
-
Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca²⁺ and cAMP concentrations in spermatozoa.PLoS One. 2012;7(2):e32354. doi: 10.1371/journal.pone.0032354. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22427794 Free PMC article.
-
The concept of allosteric interaction and its consequences for the chemistry of the brain.J Biol Chem. 2013 Sep 20;288(38):26969-26986. doi: 10.1074/jbc.X113.503375. Epub 2013 Jul 22. J Biol Chem. 2013. PMID: 23878193 Free PMC article. Review.
-
Sushi domains confer distinct trafficking profiles on GABAB receptors.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):12171-6. doi: 10.1073/pnas.1201660109. Epub 2012 Jul 9. Proc Natl Acad Sci U S A. 2012. PMID: 22778417 Free PMC article.
References
-
- Bazin H, Trinquet E, Mathis G. Time resolved amplification of cryptate emission: a versatile technology to trace biomolecular interactions. J Biotechnol. 2002;82:233–250. - PubMed
-
- Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Ther. 2006;110:533–543. - PubMed
-
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

