MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury
- PMID: 19720868
- PMCID: PMC2751981
- DOI: 10.1101/gad.1842409
MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury
Abstract
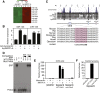
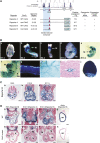
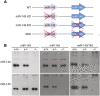
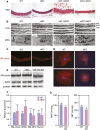
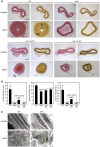
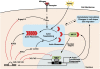
Vascular injury triggers dedifferentiation and cytoskeletal remodeling of smooth muscle cells (SMCs), culminating in vessel occlusion. Serum response factor (SRF) and its coactivator, myocardin, play a central role in the control of smooth muscle phenotypes by regulating the expression of cytoskeletal genes. We show that SRF and myocardin regulate a cardiovascular-specific microRNA (miRNA) cluster encoding miR-143 and miR-145. To assess the functions of these miRNAs in vivo, we systematically deleted them singly and in combination in mice. Mice lacking both miR-143 and miR-145 are viable and do not display overt abnormalities in smooth muscle differentiation, although they show a significant reduction in blood pressure due to reduced vascular tone. Remarkably, however, neointima formation in response to vascular injury is profoundly impeded in mice lacking these miRNAs, due to disarray of actin stress fibers and diminished migratory activity of SMCs. These abnormalities reflect the regulation of a cadre of modulators of SRF activity and actin dynamics by miR-143 and miR-145. Thus, miR-143 and miR-145 act as integral components of the regulatory network whereby SRF controls cytoskeletal remodeling and phenotypic switching of SMCs during vascular disease.
Figures

Similar articles
-
Myocardin regulates vascular response to injury through miR-24/-29a and platelet-derived growth factor receptor-β.Arterioscler Thromb Vasc Biol. 2013 Oct;33(10):2355-65. doi: 10.1161/ATVBAHA.112.301000. Epub 2013 Jul 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23825366
-
Role of miR-145 in cardiac myofibroblast differentiation.J Mol Cell Cardiol. 2014 Jan;66:94-105. doi: 10.1016/j.yjmcc.2013.08.007. Epub 2013 Aug 31. J Mol Cell Cardiol. 2014. PMID: 24001939
-
Proteasomal degradation of myocardin is required for its transcriptional activity in vascular smooth muscle cells.J Cell Physiol. 2011 Jul;226(7):1897-906. doi: 10.1002/jcp.22519. J Cell Physiol. 2011. PMID: 21506120
-
Control of smooth muscle development by the myocardin family of transcriptional coactivators.Curr Opin Genet Dev. 2004 Oct;14(5):558-66. doi: 10.1016/j.gde.2004.08.003. Curr Opin Genet Dev. 2004. PMID: 15380248 Review.
-
MicroRNA Regulatory Pathways in the Control of the Actin-Myosin Cytoskeleton.Cells. 2020 Jul 9;9(7):1649. doi: 10.3390/cells9071649. Cells. 2020. PMID: 32660059 Free PMC article. Review.
Cited by
-
MicroRNA regulation of smooth muscle gene expression and phenotype.Curr Opin Hematol. 2012 May;19(3):224-31. doi: 10.1097/MOH.0b013e3283523e57. Curr Opin Hematol. 2012. PMID: 22406821 Free PMC article. Review.
-
Regulation of vascular smooth muscle mechanotransduction by microRNAs and L-type calcium channels.Commun Integr Biol. 2013 Jan 1;6(1):e22278. doi: 10.4161/cib.22278. Commun Integr Biol. 2013. PMID: 23802033 Free PMC article.
-
Deletion of Dicer in smooth muscle affects voiding pattern and reduces detrusor contractility and neuroeffector transmission.PLoS One. 2012;7(4):e35882. doi: 10.1371/journal.pone.0035882. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558254 Free PMC article.
-
MicroRNAs in atherosclerosis and lipoprotein metabolism.Curr Opin Endocrinol Diabetes Obes. 2010 Apr;17(2):150-5. doi: 10.1097/MED.0b013e32833727a1. Curr Opin Endocrinol Diabetes Obes. 2010. PMID: 20150807 Free PMC article. Review.
-
MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function.Arterioscler Thromb Vasc Biol. 2010 Jun;30(6):1118-26. doi: 10.1161/ATVBAHA.109.200873. Epub 2010 Apr 8. Arterioscler Thromb Vasc Biol. 2010. PMID: 20378849 Free PMC article.
References
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. - PubMed
-
- Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: A component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
