Bone morphogenic protein 6: a member of a novel class of prognostic factors expressed by normal and malignant plasma cells inhibiting proliferation and angiogenesis
- PMID: 19718049
- PMCID: PMC2844406
- DOI: 10.1038/onc.2009.257
Bone morphogenic protein 6: a member of a novel class of prognostic factors expressed by normal and malignant plasma cells inhibiting proliferation and angiogenesis
Abstract
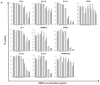
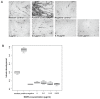
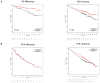
Pathogenesis of multiple myeloma is associated with an aberrant expression of pro-proliferative, pro-angiogenic and bone-metabolism-modifying factors by malignant plasma cells. Given the frequently long time span from diagnosis of early-stage plasma cell dyscrasias to overt myeloma and the mostly low proliferation rate of malignant plasma cells, we hypothesize these to similarly express a novel class of inhibitory factors of potential prognostic relevance. Bone morphogenic proteins (BMPs) represent possible candidates as they inhibit proliferation, stimulate bone formation and have an effect on the survival of cancer patients. We assessed the expression of BMPs and their receptors by Affymetrix DNA microarrays (n=779) including CD138-purified primary myeloma cell samples (n=635) of previously untreated patients. BMP6 is the only BMP expressed by malignant and normal plasma cells. Its expression is significantly lower in proliferating myeloma cells, myeloma cell lines or plasmablasts. BMP6 significantly inhibits the proliferation of myeloma cell lines, survival of primary myeloma cells and in vitro angiogenesis. A high BMP6 expression in primary myeloma cell samples delineates significantly superior overall survival for patients undergoing high-dose chemotherapy independent of conventional prognostic factors (International Staging System (ISS) stage, beta(2) microglobulin).
Conflict of interest statement
The authors declare no conflict of interests.
Figures



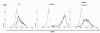




Similar articles
-
Proliferation is a central independent prognostic factor and target for personalized and risk-adapted treatment in multiple myeloma.Haematologica. 2011 Jan;96(1):87-95. doi: 10.3324/haematol.2010.030296. Epub 2010 Sep 30. Haematologica. 2011. PMID: 20884712 Free PMC article.
-
Plasma cell growth fraction using Ki-67 antigen expression identifies a subgroup of multiple myeloma patients displaying short survival within the ISS stage I.Eur J Haematol. 2007 Oct;79(4):297-304. doi: 10.1111/j.1600-0609.2007.00915.x. Epub 2007 Aug 10. Eur J Haematol. 2007. PMID: 17692103
-
Bone morphogenetic proteins and receptors are over-expressed in bone-marrow cells of multiple myeloma patients and support myeloma cells by inducing ID genes.Leuk Res. 2010 Jun;34(6):742-51. doi: 10.1016/j.leukres.2009.10.016. Epub 2009 Nov 18. Leuk Res. 2010. PMID: 19926132
-
The role of bone morphogenetic proteins in myeloma cell survival.Cytokine Growth Factor Rev. 2014 Jun;25(3):343-50. doi: 10.1016/j.cytogfr.2014.04.009. Epub 2014 May 9. Cytokine Growth Factor Rev. 2014. PMID: 24853340 Review.
-
The neural cell adhesion molecule NCAM in multiple myeloma.Leuk Lymphoma. 1996 Feb;20(5-6):389-95. doi: 10.3109/10428199609052420. Leuk Lymphoma. 1996. PMID: 8833394 Review.
Cited by
-
Inhibition of DEPDC1A, a bad prognostic marker in multiple myeloma, delays growth and induces mature plasma cell markers in malignant plasma cells.PLoS One. 2013 Apr 30;8(4):e62752. doi: 10.1371/journal.pone.0062752. Print 2013. PLoS One. 2013. PMID: 23646139 Free PMC article.
-
BMP signaling and its paradoxical effects in tumorigenesis and dissemination.Oncotarget. 2016 Nov 22;7(47):78206-78218. doi: 10.18632/oncotarget.12151. Oncotarget. 2016. PMID: 27661009 Free PMC article. Review.
-
Prospective target assessment and multimodal prediction of survival for personalized and risk-adapted treatment strategies in multiple myeloma in the GMMG-MM5 multicenter trial.J Hematol Oncol. 2019 Jun 26;12(1):65. doi: 10.1186/s13045-019-0750-5. J Hematol Oncol. 2019. PMID: 31242924 Free PMC article. Clinical Trial.
-
The glycome of normal and malignant plasma cells.PLoS One. 2013 Dec 26;8(12):e83719. doi: 10.1371/journal.pone.0083719. eCollection 2013. PLoS One. 2013. PMID: 24386263 Free PMC article.
-
Utilizing BMP-2 muteins for treatment of multiple myeloma.PLoS One. 2017 May 10;12(5):e0174884. doi: 10.1371/journal.pone.0174884. eCollection 2017. PLoS One. 2017. PMID: 28489849 Free PMC article.
References
-
- Barlogie B, Tricot G, Rasmussen E, Anaissie E, van RF, Zangari M, Fassas A, Hollmig K, Pineda-Roman M, Shaughnessy J, Epstein J, Crowley J. Blood. 2006;107:2633–2638. - PubMed
-
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Br J Haematol. 1998;102:1115–1123. - PubMed
-
- Caplan AI. J Cell Physiol. 2007;213:341–347. - PubMed
-
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. J Bone Joint Surg Am. 2003;85-A:1544–1552. - PubMed
-
- Clement JH, Sanger J, Hoffken K. Int J Cancer. 1999;80:250–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

