Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger
- PMID: 19717647
- PMCID: PMC2774559
- DOI: 10.1182/blood-2009-03-211342
Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger
Abstract
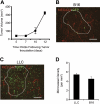
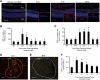
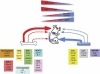
Adult bone marrow (BM) contributes to neovascularization in some but not all settings, and reasons for these discordant results have remained unexplored. We conducted novel comparative studies in which multiple neovascularization models were established in single mice to reduce variations in experimental methodology. In different combinations, BM contribution was detected in ischemic retinas and, to a lesser extent, Lewis lung carcinoma cells, whereas B16 melanomas showed little to no BM contribution. Using this spectrum of BM contribution, we demonstrate the necessity for site-specific expression of stromal-derived factor-1alpha (SDF-1alpha) and its mobilizing effects on BM. Blocking SDF-1alpha activity with neutralizing antibodies abrogated BM-derived neovascularization in lung cancer and retinopathy. Furthermore, secondary transplantation of single hematopoietic stem cells (HSCs) showed that HSCs are a long-term source of neovasculogenesis and that CD133(+)CXCR4(+) myeloid progenitor cells directly participate in new blood vessel formation in response to SDF-1alpha. The varied BM contribution seen in different model systems is suggestive of redundant mechanisms governing postnatal neovasculogenesis and provides an explanation for contradictory results observed in the field.
Figures




Similar articles
-
Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice.Exp Hematol. 2006 Aug;34(8):967-75. doi: 10.1016/j.exphem.2006.04.002. Exp Hematol. 2006. PMID: 16863903 Review.
-
Stromal cell-derived factor-1 mediates changes of bone marrow stem cells during the bone repair process.Am J Physiol Endocrinol Metab. 2016 Jan 1;310(1):E15-23. doi: 10.1152/ajpendo.00253.2015. Epub 2015 Nov 3. Am J Physiol Endocrinol Metab. 2016. PMID: 26530150
-
Characterization of endothelial progenitor cells mobilization following cutaneous wounding.Wound Repair Regen. 2010 Jul-Aug;18(4):383-90. doi: 10.1111/j.1524-475X.2010.00596.x. Epub 2010 Jun 8. Wound Repair Regen. 2010. PMID: 20546555 Free PMC article.
-
Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease.Circulation. 2004 Apr 6;109(13):1615-22. doi: 10.1161/01.CIR.0000124476.32871.E3. Epub 2004 Mar 22. Circulation. 2004. PMID: 15037527
-
CXCL12 and CXCR4 in bone marrow physiology.Expert Rev Hematol. 2010 Jun;3(3):315-22. doi: 10.1586/ehm.10.16. Expert Rev Hematol. 2010. PMID: 21082982 Review.
Cited by
-
Tumours and tissues: similar homeostatic systems?Target Oncol. 2013 Jun;8(2):97-105. doi: 10.1007/s11523-013-0277-6. Epub 2013 May 2. Target Oncol. 2013. PMID: 23636780 Review.
-
Bone marrow cell recruitment mediated by inducible nitric oxide synthase/stromal cell-derived factor-1alpha signaling repairs the acoustically damaged cochlear blood-labyrinth barrier.Am J Pathol. 2010 Dec;177(6):3089-99. doi: 10.2353/ajpath.2010.100340. Epub 2010 Nov 5. Am J Pathol. 2010. PMID: 21057001 Free PMC article.
-
History of myeloid-derived suppressor cells.Nat Rev Cancer. 2013 Oct;13(10):739-52. doi: 10.1038/nrc3581. Nat Rev Cancer. 2013. PMID: 24060865 Free PMC article. Review.
-
Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes.Circ Res. 2014 Oct 24;115(10):867-74. doi: 10.1161/CIRCRESAHA.115.304353. Epub 2014 Aug 18. Circ Res. 2014. PMID: 25136078 Free PMC article. Clinical Trial.
-
Role of connective tissue growth factor in the retinal vasculature during development and ischemia.Invest Ophthalmol Vis Sci. 2011 Nov 7;52(12):8701-10. doi: 10.1167/iovs.11-7870. Invest Ophthalmol Vis Sci. 2011. PMID: 21969300 Free PMC article.
References
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. - PubMed
-
- Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8(6):607–612. - PubMed
-
- Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7(11):1194–1201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

