Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing
- PMID: 19716852
- PMCID: PMC4289903
- DOI: 10.1016/j.jconrel.2009.08.019
Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing
Abstract
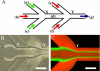
A multi-inlet microfluidic hydrodynamic focusing (MF) system to prepare lipopolyplex (LP) containing Bcl-2 antisense deoxyoligonucleotide (ODN) was developed and evaluated. The lipopolyplex nanoparticles consist of ODN:protamine:lipids (1:0.3:12.5wt/wt ratio) and the lipids included DC-Chol:egg PC:PEG-DSPE (40:58:2mol/mol%). Using K562 human erythroleukemia cells, which contain an abundance of Bcl-2 and overexpression of transferrin receptors (TfR), and G3139 (oblimerson sodium or Genasense(TM)) as a model cell line and drug, respectively, the Bcl-2 down-regulation at the mRNA and protein levels as well as cellular uptake and apoptosis was compared between the conventional bulk mixing (BM) method and the MF method. The lipopolyplex size and surface charge were characterized by dynamic light scattering (DLS) and zeta potential (zeta) measurement, respectively, while the ODN encapsulation efficiency was determined by gel electrophoresis. Cryogenic transmission electron microscopy (Cryo-TEM) was used to determine the morphology of LPs. Our results demonstrated that MF produced LP nanoparticles had similar structures but smaller size and size distribution compared to BM LP nanoparticles. MF LP nanoparticles had higher level of Bcl-2 antisense uptake and showed more efficient down-regulation of Bcl-2 protein level than BM LP nanoparticles.
Figures


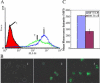

 Free ODN;
Free ODN;  BM Tf-LP, 1.0 μM ODN;
BM Tf-LP, 1.0 μM ODN;  MF LP, 1.0 μM ODN; and
MF LP, 1.0 μM ODN; and  MF Tf-LP, 1.0 μM ODN.
MF Tf-LP, 1.0 μM ODN.
 Free ODN, 1.0 μM ODN;
Free ODN, 1.0 μM ODN;  BM Tf-LP, 0.5 μM ODN;
BM Tf-LP, 0.5 μM ODN;  MF Tf-LP, 0.5 μM ODN;
MF Tf-LP, 0.5 μM ODN;  BM Tf-LP, 1.0 μM ODN; and
BM Tf-LP, 1.0 μM ODN; and  MF Tf-LP, 1.0 μM ODN.
MF Tf-LP, 1.0 μM ODN.Similar articles
-
Transferrin receptor-targeted lipid nanoparticles for delivery of an antisense oligodeoxyribonucleotide against Bcl-2.Mol Pharm. 2009 Jan-Feb;6(1):221-30. doi: 10.1021/mp800149s. Mol Pharm. 2009. PMID: 19183107 Free PMC article.
-
Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes.J Control Release. 2006 May 15;112(2):199-207. doi: 10.1016/j.jconrel.2006.02.011. Epub 2006 Mar 6. J Control Release. 2006. PMID: 16564596
-
Transferrin receptor targeted lipopolyplexes for delivery of antisense oligonucleotide g3139 in a murine k562 xenograft model.Pharm Res. 2009 Jun;26(6):1516-24. doi: 10.1007/s11095-009-9864-8. Epub 2009 Mar 17. Pharm Res. 2009. PMID: 19291371 Free PMC article.
-
Targeted delivery of antisense oligodeoxynucleotide by transferrin conjugated pH-sensitive lipopolyplex nanoparticles: a novel oligonucleotide-based therapeutic strategy in acute myeloid leukemia.Mol Pharm. 2010 Feb 1;7(1):196-206. doi: 10.1021/mp900205r. Mol Pharm. 2010. PMID: 19852511 Free PMC article.
-
Efficient delivery of an antisense oligodeoxyribonucleotide formulated in folate receptor-targeted liposomes.Anticancer Res. 2006 Mar-Apr;26(2A):1049-56. Anticancer Res. 2006. PMID: 16619505
Cited by
-
Non-coding RNAs: a key to future personalized molecular therapy?Genome Med. 2010 Feb 18;2(2):12. doi: 10.1186/gm133. Genome Med. 2010. PMID: 20236487 Free PMC article.
-
The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations.Sci Rep. 2014 Nov 19;4:7107. doi: 10.1038/srep07107. Sci Rep. 2014. PMID: 25407686 Free PMC article.
-
Targeted nanoparticles in imaging: paving the way for personalized medicine in the battle against cancer.Integr Biol (Camb). 2013 Jan;5(1):29-42. doi: 10.1039/c2ib20047c. Integr Biol (Camb). 2013. PMID: 22790418 Free PMC article. Review.
-
Microscale oral delivery devices incorporating nanoparticles.Nanomedicine (Lond). 2010 Feb;5(2):161-3. doi: 10.2217/nnm.09.113. Nanomedicine (Lond). 2010. PMID: 20148626 Free PMC article. Review. No abstract available.
-
Macrophage-Targeted Lipid Nanoparticle Delivery of microRNA-146a to Mitigate Hemorrhagic Shock-Induced Acute Respiratory Distress Syndrome.ACS Nano. 2023 Sep 12;17(17):16539-16552. doi: 10.1021/acsnano.3c01814. Epub 2023 Aug 18. ACS Nano. 2023. PMID: 37595605 Free PMC article.
References
-
- Gewirtz AM. Antisense oligonucleotide therapeutics for human leukemia. Curr. Opin. Hematol. 1998;5:59–71. - PubMed
-
- Iversen P. In vivo studies with phosphorothioate oligonucleotides. Pharmacokinet. Prologue. 1991;6(6):531–538. - PubMed
-
- Seymour LW. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit. Rev. Ther. Drug Carr. Syst. 1992;9(2):135–187. - PubMed
-
- Jahn A, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir. 2007;23(11):6289–6293. - PubMed
-
- Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech. 2004;36:381–411.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

