Yersinia pestis can bypass protective antibodies to LcrV and activation with gamma interferon to survive and induce apoptosis in murine macrophages
- PMID: 19710295
- PMCID: PMC2756853
- DOI: 10.1128/CVI.00172-09
Yersinia pestis can bypass protective antibodies to LcrV and activation with gamma interferon to survive and induce apoptosis in murine macrophages
Abstract
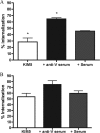
Yersinia pestis, the agent of plague, uses a type III secretion injectisome to deliver Yop proteins into macrophages to counteract phagocytosis and induce apoptosis. Additionally, internalized Y. pestis can survive in the phagosomes of naïve or gamma interferon (IFN-gamma)-activated macrophages by blocking vacuole acidification. The Y. pestis LcrV protein is a target of protective antibodies. The binding of antibodies to LcrV at the injectisome tip results in neutralization of the apoptosis of Y. pestis-infected macrophages and is used as an in vitro correlate of protective immunity. The cytokines IFN-gamma and tumor necrosis factor alpha can cooperate with anti-LcrV to promote protection against lethal Y. pestis infection in mice. It is not known if these phagocyte-activating cytokines cooperate with anti-LcrV to increase the killing of the pathogen and decrease apoptosis in macrophages. We investigated how anti-LcrV and IFN-gamma impact bacterial survival and apoptosis in cultured murine macrophages infected with Y. pestis KIM5. Y. pestis KIM5 opsonized with polyclonal or monoclonal anti-LcrV was used to infect macrophages treated with or without IFN-gamma. The phagocytosis and survival of KIM5 and the apoptosis of macrophages were measured at different time points postinfection. The results show that anti-LcrV reduced apoptosis at an early time point (5 h) but not at a later time point (24 h). Polyclonal anti-LcrV was unable to inhibit apoptosis at either time point in IFN-gamma-activated macrophages. Additionally, anti-LcrV was ineffective at promoting the killing of KIM5 in naïve or activated macrophages. We conclude that Y. pestis can bypass protective antibodies to LcrV and activation with IFN-gamma to survive and induce apoptosis in murine macrophages.
Figures
Similar articles
-
Polymorphisms in the lcrV gene of Yersinia enterocolitica and their effect on plague protective immunity.Infect Immun. 2012 Apr;80(4):1572-82. doi: 10.1128/IAI.05637-11. Epub 2012 Jan 17. Infect Immun. 2012. PMID: 22252870 Free PMC article.
-
Direct neutralization of type III effector translocation by the variable region of a monoclonal antibody to Yersinia pestis LcrV.Clin Vaccine Immunol. 2014 May;21(5):667-73. doi: 10.1128/CVI.00013-14. Epub 2014 Mar 5. Clin Vaccine Immunol. 2014. PMID: 24599533 Free PMC article.
-
Neutralization of Yersinia pestis-mediated macrophage cytotoxicity by anti-LcrV antibodies and its correlation with protective immunity in a mouse model of bubonic plague.Vaccine. 2008 Mar 20;26(13):1616-25. doi: 10.1016/j.vaccine.2008.01.033. Epub 2008 Feb 6. Vaccine. 2008. PMID: 18304706
-
Plague vaccines and the molecular basis of immunity against Yersinia pestis.Hum Vaccin. 2009 Dec;5(12):817-23. doi: 10.4161/hv.9866. Epub 2009 Dec 1. Hum Vaccin. 2009. PMID: 19786842 Review.
-
Immune defense against pneumonic plague.Immunol Rev. 2008 Oct;225:256-71. doi: 10.1111/j.1600-065X.2008.00674.x. Immunol Rev. 2008. PMID: 18837787 Free PMC article. Review.
Cited by
-
Functional assays to screen and select monoclonal antibodies that target Yersinia pestis.Hum Vaccin Immunother. 2023 Aug 1;19(2):2216085. doi: 10.1080/21645515.2023.2216085. Epub 2023 Jun 8. Hum Vaccin Immunother. 2023. PMID: 37289480 Free PMC article.
-
Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD.mBio. 2015 Feb 17;6(1):e02095-14. doi: 10.1128/mBio.02095-14. mBio. 2015. PMID: 25691590 Free PMC article.
-
Macrophage cell death upon intracellular bacterial infection.Macrophage (Houst). 2015 Apr 26;2:e779. doi: 10.14800/Macrophage.779. Macrophage (Houst). 2015. PMID: 26690967 Free PMC article.
-
A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system.Cell Host Microbe. 2010 May 20;7(5):376-87. doi: 10.1016/j.chom.2010.04.009. Cell Host Microbe. 2010. PMID: 20478539 Free PMC article.
-
Random mutagenesis identifies a C-terminal region of YopD important for Yersinia type III secretion function.PLoS One. 2015 Mar 25;10(3):e0120471. doi: 10.1371/journal.pone.0120471. eCollection 2015. PLoS One. 2015. PMID: 25807250 Free PMC article.
References
-
- Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. - PubMed
-
- Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

