SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer
- PMID: 19706487
- PMCID: PMC2736415
- DOI: 10.1073/pnas.0902042106
SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer
Abstract
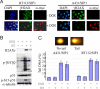
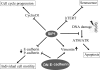
The epithelial-mesenchymal transition (EMT) contributes to cancer metastasis. Two ZEB family members, ZEB1 and ZEB2(SIP1), inhibit transcription of the E-cadherin gene and induce EMT in vitro. However, their relevance to human cancer is insufficiently studied. Here, we performed a comparative study of SIP1 and ZEB1 proteins in cancer cell lines and in one form of human malignancy, carcinoma of the bladder. Whereas ZEB1 protein was expressed in all E-cadherin-negative carcinoma cell lines, being in part responsible for the high motility of bladder cancer cells, SIP1 was hardly ever detectable in carcinoma cells in culture. However, SIP1 represented an independent factor of poor prognosis (P = 0.005) in a series of bladder cancer specimens obtained from patients treated with radiotherapy. In contrast, ZEB1 was rarely expressed in tumor tissues; and E-cadherin status did not correlate with the patients' survival. SIP1 protected cells from UV- and cisplatin-induced apoptosis in vitro but had no effect on the level of DNA damage. The anti-apoptotic effect of SIP1 was independent of either cell cycle arrest or loss of cell-cell adhesion and was associated with reduced phosphorylation of ATM/ATR targets in UV-treated cells. The prognostic value of SIP1 and its role in DNA damage response establish a link between genetic instability and metastasis and suggest a potential importance for this protein as a therapeutic target. In addition, we conclude that the nature of an EMT pathway rather than the deregulation of E-cadherin per se is critical for the progression of the disease and patients' survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

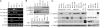

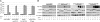
Similar articles
-
The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion.Mol Cell. 2001 Jun;7(6):1267-78. doi: 10.1016/s1097-2765(01)00260-x. Mol Cell. 2001. PMID: 11430829
-
The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2.Genes Dev. 2008 Apr 1;22(7):894-907. doi: 10.1101/gad.1640608. Genes Dev. 2008. PMID: 18381893 Free PMC article.
-
The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205.J Biol Chem. 2013 Feb 1;288(5):3275-88. doi: 10.1074/jbc.M112.408104. Epub 2012 Dec 13. J Biol Chem. 2013. PMID: 23239884 Free PMC article.
-
Role of ZEB Family Members in Proliferation, Metastasis, and Chemoresistance of Prostate Cancer Cells: Revealing Signaling Networks.Curr Cancer Drug Targets. 2021;21(9):749-767. doi: 10.2174/1568009621666210601114631. Curr Cancer Drug Targets. 2021. PMID: 34077345 Review.
-
The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer?EMBO Rep. 2010 Sep;11(9):670-7. doi: 10.1038/embor.2010.117. Epub 2010 Aug 13. EMBO Rep. 2010. PMID: 20706219 Free PMC article. Review.
Cited by
-
EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer.Oncogene. 2010 Aug 26;29(34):4741-51. doi: 10.1038/onc.2010.215. Epub 2010 Jun 7. Oncogene. 2010. PMID: 20531305 Free PMC article. Review.
-
Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes.Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15449-54. doi: 10.1073/pnas.1004900107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713713 Free PMC article.
-
Knockdown of ZEB1, a master epithelial-to-mesenchymal transition (EMT) gene, suppresses anchorage-independent cell growth of lung cancer cells.Cancer Lett. 2010 Oct 28;296(2):216-24. doi: 10.1016/j.canlet.2010.04.008. Epub 2010 May 7. Cancer Lett. 2010. PMID: 20452118 Free PMC article.
-
Co-Expression of TWIST1 and ZEB2 in Oral Squamous Cell Carcinoma Is Associated with Poor Survival.PLoS One. 2015 Jul 27;10(7):e0134045. doi: 10.1371/journal.pone.0134045. eCollection 2015. PLoS One. 2015. PMID: 26214683 Free PMC article. Clinical Trial.
-
Therapeutic Strategies Targeting Cancer Stem Cells and Their Microenvironment.Front Oncol. 2019 Oct 24;9:1104. doi: 10.3389/fonc.2019.01104. eCollection 2019. Front Oncol. 2019. PMID: 31709180 Free PMC article. Review.
References
-
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. - PubMed
-
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumor progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. - PubMed
-
- De Craene B, van Roy F, Berx G. Unraveling the signaling cascades for the Snail family of transcription factors. Cell Signal. 2005;17:535–547. - PubMed
-
- Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNA as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

