CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection
- PMID: 19692644
- PMCID: PMC2766182
- DOI: 10.4049/jimmunol.0900227
CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection
Abstract
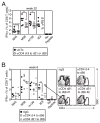
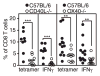
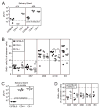
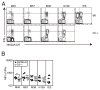
Murine CMV (MCMV) establishes a systemic, low-level persistent infection resulting in the accumulation of CD8(+) T cells specific for a subset of viral epitopes, a process called memory inflation. Although replicating virus is rarely detected in chronically infected C57BL/6 mice, these inflationary cells display a phenotype suggestive of repeated Ag stimulation, and they remain functional. CD4(+) T cells have been implicated in maintaining the function and/or number of CD8(+) T cells in other chronic infections. Moreover, CD4(+) T cells are essential for complete control of MCMV. Thus, we wondered whether CD4(+) T cell deficiency would result in impaired MCMV-specific CD8(+) T cell responses. Here we show that CD4(+) T cell deficiency had an epitope-specific impact on CD8(+) T cell memory inflation. Of the three codominant T cell responses during chronic infection, only accumulation of the late-appearing IE3-specific CD8(+) T cells was substantially impaired in CD4(+) T cell-deficient mice. Moreover, the increased viral activity did not drive increased CD8(+) T cell division or substantial dysfunction in any MCMV-specific population that we studied. These data show that CD4(+) T cell help is needed for inflation of a response that develops only during chronic infection but is otherwise dispensable for the steady state maintenance and function of MCMV-specific CD8(+) T cells.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





Similar articles
-
Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus.PLoS Pathog. 2011 Oct;7(10):e1002295. doi: 10.1371/journal.ppat.1002295. Epub 2011 Oct 6. PLoS Pathog. 2011. PMID: 21998590 Free PMC article.
-
Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection.J Immunol. 2006 Jul 1;177(1):450-8. doi: 10.4049/jimmunol.177.1.450. J Immunol. 2006. PMID: 16785542
-
Stochastic Expansions Maintain the Clonal Stability of CD8+ T Cell Populations Undergoing Memory Inflation Driven by Murine Cytomegalovirus.J Immunol. 2020 Jan 1;204(1):112-121. doi: 10.4049/jimmunol.1900455. Epub 2019 Dec 9. J Immunol. 2020. PMID: 31818981 Free PMC article.
-
MHC class I immune evasion in MCMV infection.Med Microbiol Immunol. 2008 Jun;197(2):191-204. doi: 10.1007/s00430-008-0089-y. Epub 2008 Mar 11. Med Microbiol Immunol. 2008. PMID: 18330598 Review.
-
Fuel and brake of memory T cell inflation.Med Microbiol Immunol. 2019 Aug;208(3-4):329-338. doi: 10.1007/s00430-019-00587-9. Epub 2019 Mar 9. Med Microbiol Immunol. 2019. PMID: 30852648 Review.
Cited by
-
IL-10 suppression of NK/DC crosstalk leads to poor priming of MCMV-specific CD4 T cells and prolonged MCMV persistence.PLoS Pathog. 2012;8(8):e1002846. doi: 10.1371/journal.ppat.1002846. Epub 2012 Aug 2. PLoS Pathog. 2012. PMID: 22876184 Free PMC article.
-
Antiviral T cell response triggers cytomegalovirus hepatitis in mice.J Virol. 2012 Dec;86(23):12879-90. doi: 10.1128/JVI.01752-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993151 Free PMC article.
-
Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything.J Intern Med. 2010 May;267(5):483-501. doi: 10.1111/j.1365-2796.2010.02220.x. J Intern Med. 2010. PMID: 20433576 Free PMC article. Review.
-
Memory Inflation Drives Tissue-Resident Memory CD8+ T Cell Maintenance in the Lung After Intranasal Vaccination With Murine Cytomegalovirus.Front Immunol. 2018 Aug 14;9:1861. doi: 10.3389/fimmu.2018.01861. eCollection 2018. Front Immunol. 2018. PMID: 30154789 Free PMC article.
-
Buffered memory: a hypothesis for the maintenance of functional, virus-specific CD8(+) T cells during cytomegalovirus infection.Immunol Res. 2011 Dec;51(2-3):195-204. doi: 10.1007/s12026-011-8251-9. Immunol Res. 2011. PMID: 22058020 Review.
References
-
- Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. - PubMed
-
- Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

