Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial
- PMID: 19690306
- PMCID: PMC3657724
- DOI: 10.1001/jama.2009.1198
Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial
Abstract
Context: There are few randomized controlled trials on the effectiveness of palliative care interventions to improve the care of patients with advanced cancer.
Objective: To determine the effect of a nursing-led intervention on quality of life, symptom intensity, mood, and resource use in patients with advanced cancer.
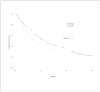
Design, setting, and participants: Randomized controlled trial conducted from November 2003 through May 2008 of 322 patients with advanced cancer in a rural, National Cancer Institute-designated comprehensive cancer center in New Hampshire and affiliated outreach clinics and a VA medical center in Vermont.
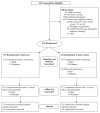
Interventions: A multicomponent, psychoeducational intervention (Project ENABLE [Educate, Nurture, Advise, Before Life Ends]) conducted by advanced practice nurses consisting of 4 weekly educational sessions and monthly follow-up sessions until death or study completion (n = 161) vs usual care (n = 161).
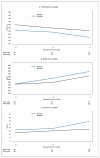
Main outcome measures: Quality of life was measured by the Functional Assessment of Chronic Illness Therapy for Palliative Care (score range, 0-184). Symptom intensity was measured by the Edmonton Symptom Assessment Scale (score range, 0-900). Mood was measured by the Center for Epidemiological Studies Depression Scale (range, 0-60). These measures were assessed at baseline, 1 month, and every 3 months until death or study completion. Intensity of service was measured as the number of days in the hospital and in the intensive care unit (ICU) and the number of emergency department visits recorded in the electronic medical record.
Results: A total of 322 participants with cancer of the gastrointestinal tract (41%; 67 in the usual care group vs 66 in the intervention group), lung (36%; 58 vs 59), genitourinary tract (12%; 20 vs 19), and breast (10%; 16 vs 17) were randomized. The estimated treatment effects (intervention minus usual care) for all participants were a mean (SE) of 4.6 (2) for quality of life (P = .02), -27.8 (15) for symptom intensity (P = .06), and -1.8 (0.81) for depressed mood (P = .02). The estimated treatment effects in participants who died during the study were a mean (SE) of 8.6 (3.6) for quality of life (P = .02), -24.2 (20.5) for symptom intensity (P = .24), and -2.7 (1.2) for depressed mood (P = .03). Intensity of service did not differ between the 2 groups.
Conclusion: Compared with participants receiving usual oncology care, those receiving a nurse-led, palliative care-focused intervention addressing physical, psychosocial, and care coordination provided concurrently with oncology care had higher scores for quality of life and mood, but did not have improvements in symptom intensity scores or reduced days in the hospital or ICU or emergency department visits.
Trial registration: clinicaltrials.gov Identifier: NCT00253383.
Figures

Comment in
-
Study design and palliative care intervention.JAMA. 2009 Dec 16;302(23):2551; author reply 2551. doi: 10.1001/jama.2009.1828. JAMA. 2009. PMID: 20009052 No abstract available.
-
Nurse-led multicomponent psychoeducational palliative intervention improves quality of life and mood in patients with advanced cancer.Evid Based Nurs. 2010 Feb;13(1):9-10. doi: 10.1136/ebn1007. Evid Based Nurs. 2010. PMID: 20179053 No abstract available.
Similar articles
-
Effect of an Early Palliative Care Telehealth Intervention vs Usual Care on Patients With Heart Failure: The ENABLE CHF-PC Randomized Clinical Trial.JAMA Intern Med. 2020 Sep 1;180(9):1203-1213. doi: 10.1001/jamainternmed.2020.2861. JAMA Intern Med. 2020. PMID: 32730613 Free PMC article. Clinical Trial.
-
Effect of an Oncology Nurse-Led Primary Palliative Care Intervention on Patients With Advanced Cancer: The CONNECT Cluster Randomized Clinical Trial.JAMA Intern Med. 2021 Nov 1;181(11):1451-1460. doi: 10.1001/jamainternmed.2021.5185. JAMA Intern Med. 2021. PMID: 34515737 Free PMC article. Clinical Trial.
-
The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions.Palliat Support Care. 2009 Mar;7(1):75-86. doi: 10.1017/S1478951509000108. Palliat Support Care. 2009. PMID: 19619377 Free PMC article. Clinical Trial.
-
Early palliative care for adults with advanced cancer.Cochrane Database Syst Rev. 2017 Jun 12;6(6):CD011129. doi: 10.1002/14651858.CD011129.pub2. Cochrane Database Syst Rev. 2017. PMID: 28603881 Free PMC article. Review.
-
Educational interventions for the management of cancer-related fatigue in adults.Cochrane Database Syst Rev. 2016 Nov 24;11(11):CD008144. doi: 10.1002/14651858.CD008144.pub2. Cochrane Database Syst Rev. 2016. PMID: 27883365 Free PMC article. Review.
Cited by
-
Clinical trial of a supportive care team for patients with advanced cancer.J Pain Symptom Manage. 2013 Dec;46(6):775-84. doi: 10.1016/j.jpainsymman.2012.12.008. Epub 2013 Mar 22. J Pain Symptom Manage. 2013. PMID: 23523362 Free PMC article. Clinical Trial.
-
Facilitating communication for critically ill patients and their family members: Study protocol for two randomized trials implemented in the U.S. and France.Contemp Clin Trials. 2021 Aug;107:106465. doi: 10.1016/j.cct.2021.106465. Epub 2021 Jun 3. Contemp Clin Trials. 2021. PMID: 34091062 Free PMC article.
-
Joint modeling quality of life and survival using a terminal decline model in palliative care studies.Stat Med. 2013 Apr 15;32(8):1394-406. doi: 10.1002/sim.5635. Epub 2012 Sep 23. Stat Med. 2013. PMID: 23001893 Free PMC article. Clinical Trial.
-
Palliative and end-of-life care research: embracing new opportunities.Nurs Outlook. 2012 Nov-Dec;60(6):384-90. doi: 10.1016/j.outlook.2012.08.006. Nurs Outlook. 2012. PMID: 23141198 Free PMC article. Review.
-
Palliative care: an approach for all internists: comment on "Early palliative care in advanced lung cancer: a qualitative study".JAMA Intern Med. 2013 Feb 25;173(4):291-2. doi: 10.1001/jamainternmed.2013.1888. JAMA Intern Med. 2013. PMID: 23358724 Free PMC article. No abstract available.
References
-
- American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007.
-
- Field MJ, Cassel CK. Approaching Death: Improving Care at the End of Life. Washington, D.C: National Academy Press; 1997. - PubMed
-
- Foley KM, Gelband H. Improving Palliative Care for Cancer. Washington, D.C: Institute of Medicine and National Research Council; 2001. - PubMed
-
- Morrison RS, Meier DE. Clinical practice. Palliative care. N Engl J Med. 2004 Jun 17;350(25):2582–2590. - PubMed
-
- World Health Organization. [Accessed December, 2005];Palliative Care: What is it. www.who.org.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

