Chemical interrogation of FOXO3a nuclear translocation identifies potent and selective inhibitors of phosphoinositide 3-kinases
- PMID: 19690175
- PMCID: PMC2788888
- DOI: 10.1074/jbc.M109.038984
Chemical interrogation of FOXO3a nuclear translocation identifies potent and selective inhibitors of phosphoinositide 3-kinases
Abstract
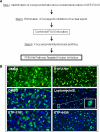
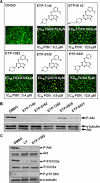
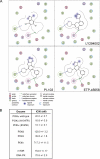
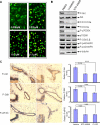
Activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway is one the most frequent genetic events in human cancer. A cell-based imaging assay that monitored the translocation of the Akt effector protein, Forkhead box O (FOXO), from the cytoplasm to the nucleus was employed to screen a collection of 33,992 small molecules. The positive compounds were used to screen kinases known to be involved in FOXO translocation. Pyrazolopyrimidine derivatives were found to be potent FOXO relocators as well as biochemical inhibitors of PI3Kalpha. A combination of virtual screening and molecular modeling led to the development of a structure-activity relationship, which indicated the preferred substituents on the pyrazolopyrimidine scaffold. This leads to the synthesis of ETP-45658, which is a potent and selective inhibitor of phosphoinositide 3-kinases and demonstrates mechanism of action in tumor cell lines and in vivo in treated mice.
Figures
Similar articles
-
A novel phosphatidylinositol 3-kinase (PI3K) inhibitor directs a potent FOXO-dependent, p53-independent cell cycle arrest phenotype characterized by the differential induction of a subset of FOXO-regulated genes.Breast Cancer Res. 2014 Dec 9;16(6):482. doi: 10.1186/s13058-014-0482-y. Breast Cancer Res. 2014. PMID: 25488803 Free PMC article.
-
Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening.Chembiochem. 2008 Sep 22;9(14):2229-37. doi: 10.1002/cbic.200800255. Chembiochem. 2008. PMID: 18756565
-
The PI3K/Akt/FOXO3a/p27Kip1 signaling contributes to anti-inflammatory drug-suppressed proliferation of human osteoblasts.Biochem Pharmacol. 2010 Mar 15;79(6):926-37. doi: 10.1016/j.bcp.2009.10.019. Epub 2009 Oct 31. Biochem Pharmacol. 2010. PMID: 19883628
-
Activated phosphatidylinositol 3-kinase/Akt inhibits the transition of endothelial progenitor cells to mesenchymal cells by regulating the forkhead box subgroup O-3a signaling.Cell Physiol Biochem. 2015;35(4):1643-53. doi: 10.1159/000373978. Epub 2015 Mar 18. Cell Physiol Biochem. 2015. PMID: 25824462
-
FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma.Cancer Res. 2013 Apr 1;73(7):2189-98. doi: 10.1158/0008-5472.CAN-12-3767. Epub 2013 Feb 1. Cancer Res. 2013. PMID: 23378341
Cited by
-
Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer.Nat Rev Drug Discov. 2013 Jan;12(1):51-63. doi: 10.1038/nrd3683. Nat Rev Drug Discov. 2013. PMID: 23274471 Review.
-
Use of Microfluidics for Study of FOXO3 Translocation Dynamics.Methods Mol Biol. 2025;2871:155-161. doi: 10.1007/978-1-0716-4217-7_14. Methods Mol Biol. 2025. PMID: 39565586
-
A novel phosphatidylinositol 3-kinase (PI3K) inhibitor directs a potent FOXO-dependent, p53-independent cell cycle arrest phenotype characterized by the differential induction of a subset of FOXO-regulated genes.Breast Cancer Res. 2014 Dec 9;16(6):482. doi: 10.1186/s13058-014-0482-y. Breast Cancer Res. 2014. PMID: 25488803 Free PMC article.
-
Methylseleninic acid promotes antitumour effects via nuclear FOXO3a translocation through Akt inhibition.Pharmacol Res. 2015 Dec;102:218-34. doi: 10.1016/j.phrs.2015.09.009. Epub 2015 Nov 4. Pharmacol Res. 2015. PMID: 26375988 Free PMC article.
-
Discovery of a Novel, Isothiazolonaphthoquinone-Based Small Molecule Activator of FOXO Nuclear-Cytoplasmic Shuttling.PLoS One. 2016 Dec 9;11(12):e0167491. doi: 10.1371/journal.pone.0167491. eCollection 2016. PLoS One. 2016. PMID: 27936162 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

