Phosphoinositide signaling: new tools and insights
- PMID: 19675354
- PMCID: PMC3126675
- DOI: 10.1152/physiol.00014.2009
Phosphoinositide signaling: new tools and insights
Abstract
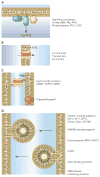
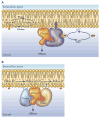
Phosphoinositides constitute only a small fraction of cellular phospholipids, yet their importance in the regulation of cellular functions can hardly be overstated. The rapid metabolic response of phosphoinositides after stimulation of certain cell surface receptors was the first indication that these lipids could serve as regulatory molecules. These early observations opened research areas that ultimately clarified the plasma membrane role of phosphoinositides in Ca(2+) signaling. However, research of the last 10 years has revealed a much broader range of processes dependent on phosphoinositides. These lipids control organelle biology by regulating vesicular trafficking, and they modulate lipid distribution and metabolism more generally via their close relationship with lipid transfer proteins. Phosphoinositides also regulate ion channels, pumps, and transporters as well as both endocytic and exocytic processes. The significance of phosphoinositides found within the nucleus is still poorly understood, and a whole new research concerns the highly phosphorylated inositols that also appear to control multiple nuclear processes. The expansion of research and interest in phosphoinositides naturally created a demand for new approaches to determine where, within the cell, these lipids exert their effects. Imaging of phosphoinositide dynamics within live cells has become a standard cell biological method. These new tools not only helped us localize phosphoinositides within the cell but also taught us how tightly phosphoinositide control can be linked with distinct effector protein complexes. The recent progress allows us to understand the underlying causes of certain human diseases and design new strategies for therapeutic interventions.
Figures
Similar articles
-
Phosphoinositides: tiny lipids with giant impact on cell regulation.Physiol Rev. 2013 Jul;93(3):1019-137. doi: 10.1152/physrev.00028.2012. Physiol Rev. 2013. PMID: 23899561 Free PMC article. Review.
-
Regulation of Ca2+ entry by inositol lipids in mammalian cells by multiple mechanisms.Cell Calcium. 2009 Jun;45(6):527-34. doi: 10.1016/j.ceca.2009.03.013. Epub 2009 Apr 22. Cell Calcium. 2009. PMID: 19395084 Free PMC article. Review.
-
Phosphoinositides: multipurpose cellular lipids with emerging roles in cell death.Cell Death Differ. 2019 May;26(5):781-793. doi: 10.1038/s41418-018-0269-2. Epub 2019 Feb 11. Cell Death Differ. 2019. PMID: 30742090 Free PMC article. Review.
-
Phosphoinositide control of membrane protein function: a frontier led by studies on ion channels.Annu Rev Physiol. 2015;77:81-104. doi: 10.1146/annurev-physiol-021113-170358. Epub 2014 Oct 2. Annu Rev Physiol. 2015. PMID: 25293526 Free PMC article. Review.
-
Phosphoinositides and vesicular membrane traffic.Biochim Biophys Acta. 2012 Aug;1821(8):1104-13. doi: 10.1016/j.bbalip.2012.01.002. Epub 2012 Jan 14. Biochim Biophys Acta. 2012. PMID: 22281700 Free PMC article. Review.
Cited by
-
Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling.Proc Natl Acad Sci U S A. 2010 May 4;107(18):8225-30. doi: 10.1073/pnas.1000157107. Epub 2010 Apr 19. Proc Natl Acad Sci U S A. 2010. PMID: 20404150 Free PMC article.
-
STX2 Promotes Trophoblast Growth, Migration, and Invasion Through Activation of the PI3K-AKT Pathway in Preeclampsia.Front Cell Dev Biol. 2021 Jul 6;9:615973. doi: 10.3389/fcell.2021.615973. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34295885 Free PMC article.
-
Exploring the Role of PI3P in Platelets: Insights from a Novel External PI3P Pool.Biomolecules. 2023 Mar 24;13(4):583. doi: 10.3390/biom13040583. Biomolecules. 2023. PMID: 37189331 Free PMC article.
-
Inositol lipid regulation of lipid transfer in specialized membrane domains.Trends Cell Biol. 2013 Jun;23(6):270-8. doi: 10.1016/j.tcb.2013.01.009. Epub 2013 Mar 13. Trends Cell Biol. 2013. PMID: 23489878 Free PMC article. Review.
-
Heparin-Binding Hemagglutinin Adhesin (HBHA) Is Involved in Intracytosolic Lipid Inclusions Formation in Mycobacteria.Front Microbiol. 2018 Sep 24;9:2258. doi: 10.3389/fmicb.2018.02258. eCollection 2018. Front Microbiol. 2018. PMID: 30333800 Free PMC article.
References
-
- Aikawa Y, Martin TF. ADP-ribosylation factor 6 regulation of phosphatidylinositol-4,5-bisphosphate synthesis, endocytosis, and exocytosis. Methods Enzymol. 2005;404:422–431. - PubMed
-
- Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. - PubMed
-
- Astle MV, Seaton G, Davies EM, Fedele CG, Rahman P, Arsala L, Mitchell CA. Regulation of phosphoinositide signaling by the inositol polyphosphate 5-phosphatases. IUBMB Life. 2006;58:451–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

