Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents
- PMID: 19670441
- PMCID: PMC3755738
- DOI: 10.1002/ana.21673
Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents
Abstract
Objective: Activation of metabotropic glutamate receptor 5 (mGluR5) has neuroprotective properties in vitro and has been reported to limit postischemic lesion volume in vivo. Previously, mGluR5 has been identified on microglia in vitro, but the effects of mGluR5 activation on inflammation in vivo or on recovery after spinal cord injury is unknown.
Methods: Rats received intrathecal infusion of the selective mGluR5 agonist (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) for 7 days after moderate impact spinal cord injury at T9. Complementary studies examined CHPG effects on activated spinal microglia cultures.
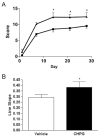
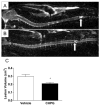
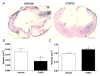
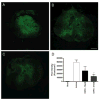
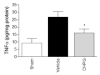
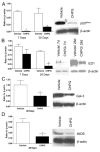
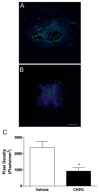
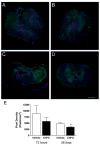
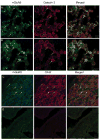
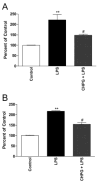
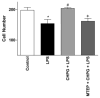
Results: Functional motor recovery was significantly increased by CHPG treatment up to 28 days after injury, with improvements in weight bearing, step taking, and coordination of stepping behavior. CHPG treatment significantly reduced lesion volume and increased white matter sparing at 28 days after injury. Administration of CHPG attenuated microglial-associated inflammatory responses in a dose-dependent fashion, including expression of ED1, Iba-1, Galectin-3, NADPH oxidase components, tumor necrosis factor-alpha, and inducible nitric oxide synthase. Because mGluR5 is expressed by microglial cells in the rat spinal cord, such effects may be mediated by direct action on microglial cells. mGluR5 stimulation also reduced microglial activation and decreased microglial-induced neurotoxicity in spinal cord microglia cultures; the latter effects were blocked by the selective mGluR5 antagonist MTEP.
Interpretation: These data demonstrate that mGluR5 activation can reduce microglial-associated inflammation, suggesting that the protective effects of mGluR5 agonists may reflect this action. Ann Neurol 2009;66:63-74.
Conflict of interest statement
Potential conflict of interest: Drs. Faden and Byrnes are co-inventors on a patent application that has been filed by Georgetown University related to the technology that is described in this paper.
Figures
Similar articles
-
Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury.J Neuroinflammation. 2012 Feb 28;9:43. doi: 10.1186/1742-2094-9-43. J Neuroinflammation. 2012. PMID: 22373400 Free PMC article.
-
Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase.J Biol Chem. 2009 Jun 5;284(23):15629-39. doi: 10.1074/jbc.M806139200. Epub 2009 Apr 13. J Biol Chem. 2009. PMID: 19364772 Free PMC article.
-
Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity.Glia. 2009 Apr 1;57(5):550-60. doi: 10.1002/glia.20783. Glia. 2009. PMID: 18816644 Free PMC article.
-
Metabotropic glutamate receptors as targets for multipotential treatment of neurological disorders.Neurotherapeutics. 2009 Jan;6(1):94-107. doi: 10.1016/j.nurt.2008.10.038. Neurotherapeutics. 2009. PMID: 19110202 Free PMC article. Review.
-
Emerging Roles of Microglia Depletion in the Treatment of Spinal Cord Injury.Cells. 2022 Jun 9;11(12):1871. doi: 10.3390/cells11121871. Cells. 2022. PMID: 35741000 Free PMC article. Review.
Cited by
-
The role of the microglia in acute CNS injury.Metab Brain Dis. 2015 Apr;30(2):381-92. doi: 10.1007/s11011-014-9531-6. Epub 2014 Mar 29. Metab Brain Dis. 2015. PMID: 24682762 Free PMC article. Review.
-
Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration, and alters microglial polarization after experimental traumatic brain injury.Neurotherapeutics. 2014 Oct;11(4):857-69. doi: 10.1007/s13311-014-0298-6. Neurotherapeutics. 2014. PMID: 25096154 Free PMC article.
-
The Physio-Pathological Role of Group I Metabotropic Glutamate Receptors Expressed by Microglia in Health and Disease with a Focus on Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2023 Mar 9;24(6):5240. doi: 10.3390/ijms24065240. Int J Mol Sci. 2023. PMID: 36982315 Free PMC article. Review.
-
Activation of mGluR5 Attenuates Microglial Activation and Neuronal Apoptosis in Early Brain Injury After Experimental Subarachnoid Hemorrhage in Rats.Neurochem Res. 2015 Jun;40(6):1121-32. doi: 10.1007/s11064-015-1572-7. Epub 2015 Apr 7. Neurochem Res. 2015. PMID: 25846008
-
Grb2 Expression in Acute Spinal Cord Injury After Methylprednisolone Intrathecal Injection in Rats.Int J Spine Surg. 2023 Oct;17(5):678-683. doi: 10.14444/8527. Epub 2023 Oct 26. Int J Spine Surg. 2023. PMID: 37884335 Free PMC article.
References
-
- Dumont RJ, Okonkwo DO, Verma S, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. - PubMed
-
- Tator CH. Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med. 1996;19:206–214. - PubMed
-
- Popovich PG, Guan Z, McGaughy V, et al. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623– 633. - PubMed
-
- Keane RW, Davis AR, Dietrich WD. Inflammatory and apoptotic signaling after spinal cord injury. J Neurotrauma. 2006;23:335–344. - PubMed
-
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

