Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial
- PMID: 19667268
- PMCID: PMC2744271
- DOI: 10.1200/JCO.2008.21.6887
Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial
Abstract
Purpose: We assessed Tau protein expression in primary breast cancer specimens of patients included in the National Surgical Breast and Bowel Project (NSABP) -B 28 clinical trial. Expression levels were correlated with disease-free survival (DFS) and overall survival (OS). Interaction between this marker and paclitaxel efficacy was also examined.
Methods: Tissue microarrays were available for 1,942 patients (63% of all trial participants) who were randomly assigned to receive either four courses of doxorubicin and cyclophosphamide (AC) or AC followed by four courses of adjuvant paclitaxel chemotherapy. All patients who were hormone receptor positive received adjuvant endocrine therapy. Immunohistochemistry was used to measure Tau expression at M. D. Anderson Cancer Center blinded to clinical outcome. Correlation between Tau and OS and DFS was performed by the NSABP Biostatistical Center.
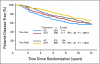
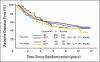
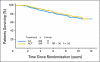
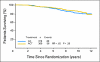
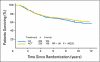
Results: Forty-three percent of patients were Tau positive. Tau positivity correlated with estrogen receptor (ER) -positive status (P < .0001), lower histologic grade (P < .001), and lack of human epidermal growth factor receptor 2 (HER2) expression (P < .0001). In univariate analyses, Tau- and ER-positive status were both associated with better DFS and OS (P < .0001) whereas greater tumor size, high grade, lymph node- and HER2-positive status were each associated with worse DFS and OS (P < .0001). In multivariate analysis, the same variables except HER2 remained significant. There was no significant interaction between Tau expression and benefit from paclitaxel in the total population or among ER-positive or -negative patients.
Conclusion: High Tau protein expression is associated with better prognosis in patients treated with adjuvant anthracycline and paclitaxel chemotherapy and endocrine therapy, but there was no significant interaction between Tau expression and paclitaxel benefit.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



Similar articles
-
Gene expression of estrogen receptor, progesterone receptor and microtubule-associated protein Tau in high-risk early breast cancer: a quest for molecular predictors of treatment benefit in the context of a Hellenic Cooperative Oncology Group trial.Breast Cancer Res Treat. 2009 Jul;116(1):131-43. doi: 10.1007/s10549-008-0144-9. Epub 2008 Jul 31. Breast Cancer Res Treat. 2009. PMID: 18668363 Clinical Trial.
-
Assessment of microtubule-associated protein (MAP)-Tau expression as a predictive and prognostic marker in TACT; a trial assessing substitution of sequential docetaxel for FEC as adjuvant chemotherapy for early breast cancer.Breast Cancer Res Treat. 2014 Apr;144(2):331-41. doi: 10.1007/s10549-014-2855-4. Epub 2014 Feb 12. Breast Cancer Res Treat. 2014. PMID: 24519386 Clinical Trial.
-
21-Gene Recurrence Score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: results from NSABP B-28/NRG Oncology.Breast Cancer Res Treat. 2018 Feb;168(1):69-77. doi: 10.1007/s10549-017-4550-8. Epub 2017 Nov 11. Breast Cancer Res Treat. 2018. PMID: 29128898 Free PMC article. Clinical Trial.
-
Effects of Estrogen Receptor and Human Epidermal Growth Factor Receptor-2 Levels on the Efficacy of Trastuzumab: A Secondary Analysis of the HERA Trial.JAMA Oncol. 2016 Aug 1;2(8):1040-7. doi: 10.1001/jamaoncol.2016.0339. JAMA Oncol. 2016. PMID: 27100299
-
Adjuvant chemotherapy in early breast cancer.Dan Med J. 2016 May;63(5):B5222. Dan Med J. 2016. PMID: 27127018 Review.
Cited by
-
Predictive and prognostic values of Tau and BubR1 protein in prostate cancer and their relationship to the Gleason score.Med Oncol. 2013 Jun;30(2):526. doi: 10.1007/s12032-013-0526-7. Epub 2013 Mar 9. Med Oncol. 2013. PMID: 23475578
-
Systemic therapy for early-stage breast cancer: learning from the past to build the future.Nat Rev Clin Oncol. 2022 Dec;19(12):763-774. doi: 10.1038/s41571-022-00687-1. Epub 2022 Oct 17. Nat Rev Clin Oncol. 2022. PMID: 36253451 Free PMC article. Review.
-
Dichotomous role of microtubule associated protein tau as a biomarker of response to and a target for increasing efficacy of taxane treatment in cancers of epithelial origin.Pharmacol Res. 2021 Jun;168:105585. doi: 10.1016/j.phrs.2021.105585. Epub 2021 Mar 30. Pharmacol Res. 2021. PMID: 33798735 Free PMC article. Review.
-
Measurement of molecular biomarkers that predict the tumor response in estrogen receptor-positive breast cancers after dose-dense (biweekly) paclitaxel/carboplatin neoadjuvant chemotherapy.Oncotarget. 2017 Jul 28;8(60):101087-101094. doi: 10.18632/oncotarget.19686. eCollection 2017 Nov 24. Oncotarget. 2017. PMID: 29254147 Free PMC article.
-
Tau expression correlated with breast cancer sensitivity to taxanes-based neoadjuvant chemotherapy.Tumour Biol. 2013 Feb;34(1):33-8. doi: 10.1007/s13277-012-0507-z. Epub 2012 Sep 14. Tumour Biol. 2013. PMID: 22976542
References
-
- Hess KR, Anderson K, Symmans WF, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24:4236–4244. - PubMed
-
- Peintinger F, Anderson K, Mazouni C, et al. Thirty-gene pharmacogenomic test correlates with residual cancer burden after preoperative chemotherapy breast cancer. Clin Cancer Res. 2007;13:4078–4082. - PubMed
-
- Wagner P, Wang B, Clark E, et al. Microtubule associated protein (MAP)-tau: A novel mediator of paclitaxel sensitivity in vitro and in vivo. Cell Cycle. 2005;4:1149–1152. - PubMed
-
- Jimeno A, Hallur G, Chan A, et al. Development of two novel benzoylphenylurea sulfur analogues and evidence that the microtubule-associated protein tau is predictive of their activity in pancreatic cancer. Mol Cancer Ther. 2007;6:1509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

