Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue
- PMID: 19667202
- PMCID: PMC2732798
- DOI: 10.1073/pnas.0906267106
Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue
Abstract
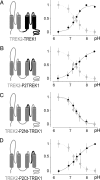
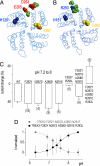
Mechanosensitive K(+) channels TREK1 and TREK2 form a subclass of two P-domain K(+) channels. They are potently activated by polyunsaturated fatty acids and are involved in neuroprotection, anesthesia, and pain perception. Here, we show that acidification of the extracellular medium strongly inhibits TREK1 with an apparent pK near to 7.4 corresponding to the physiological pH. The all-or-none effect of pH variation is steep and is observed within one pH unit. TREK2 is not inhibited but activated by acidification within the same range of pH, despite its close homology with TREK1. A single conserved residue, H126 in TREK1 and H151 in TREK2, is involved in proton sensing. This histidine is located in the M1P1 extracellular loop preceding the first P domain. The differential effect of acidification, that is, activation for TREK2 and inhibition for TREK1, involves other residues located in the P2M4 loop, linking the second P domain and the fourth membrane-spanning segment. Structural modeling of TREK1 and TREK2 and site-directed mutagenesis strongly suggest that attraction or repulsion between the protonated side chain of histidine and closely located negatively or positively charged residues in P2M4 control outer gating of these channels. The differential sensitivity of TREK1 and TREK2 to external pH variations discriminates between these two K(+) channels that otherwise share the same regulations by physical and chemical stimuli, and by hormones and neurotransmitters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels.Proc Natl Acad Sci U S A. 2016 Apr 12;113(15):4194-9. doi: 10.1073/pnas.1522459113. Epub 2016 Mar 28. Proc Natl Acad Sci U S A. 2016. PMID: 27035963 Free PMC article.
-
Neuronal microRNAs modulate TREK two-pore domain K+ channel expression and current density.RNA Biol. 2020 May;17(5):651-662. doi: 10.1080/15476286.2020.1722450. Epub 2020 Feb 10. RNA Biol. 2020. PMID: 31994436 Free PMC article.
-
Ischaemic concentrations of lactate increase TREK1 channel activity by interacting with a single histidine residue in the carboxy terminal domain.J Physiol. 2016 Jan 1;594(1):59-81. doi: 10.1113/JP270706. Epub 2015 Nov 17. J Physiol. 2016. PMID: 26445100 Free PMC article.
-
How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2.Ann N Y Acad Sci. 2015 Sep;1352:20-32. doi: 10.1111/nyas.12874. Epub 2015 Aug 31. Ann N Y Acad Sci. 2015. PMID: 26332952 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Two-pore Domain Potassium Channels in Astrocytes.Exp Neurobiol. 2016 Oct;25(5):222-232. doi: 10.5607/en.2016.25.5.222. Epub 2016 Oct 26. Exp Neurobiol. 2016. PMID: 27790056 Free PMC article. Review.
-
The isoforms generated by alternative translation initiation adopt similar conformation in the selectivity filter in TREK-2.J Physiol Biochem. 2015 Dec;71(4):601-10. doi: 10.1007/s13105-015-0422-z. Epub 2015 Aug 14. J Physiol Biochem. 2015. PMID: 26271386
-
Mechanism of external K+ sensitivity of KCNQ1 channels.J Gen Physiol. 2023 May 1;155(5):e202213205. doi: 10.1085/jgp.202213205. Epub 2023 Feb 21. J Gen Physiol. 2023. PMID: 36809486 Free PMC article.
-
The pore structure and gating mechanism of K2P channels.EMBO J. 2011 Aug 5;30(17):3607-19. doi: 10.1038/emboj.2011.268. EMBO J. 2011. PMID: 21822218 Free PMC article.
-
Physical basis for distinct basal and mechanically gated activity of the human K+ channel TRAAK.Neuron. 2021 Sep 15;109(18):2902-2913.e4. doi: 10.1016/j.neuron.2021.07.009. Epub 2021 Aug 13. Neuron. 2021. PMID: 34390650 Free PMC article.
References
-
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. - PubMed
-
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

