Variations in the colchicine-binding domain provide insight into the structural switch of tubulin
- PMID: 19666559
- PMCID: PMC2728970
- DOI: 10.1073/pnas.0904223106
Variations in the colchicine-binding domain provide insight into the structural switch of tubulin
Abstract
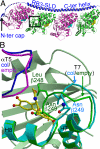
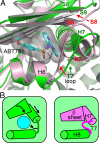
Structural changes occur in the alphabeta-tubulin heterodimer during the microtubule assembly/disassembly cycle. Their most prominent feature is a transition from a straight, microtubular structure to a curved structure. There is a broad range of small molecule compounds that disturbs the microtubule cycle, a class of which targets the colchicine-binding site and prevents microtubule assembly. This class includes compounds with very different chemical structures, and it is presently unknown whether they prevent tubulin polymerization by the same mechanism. To address this issue, we have determined the structures of tubulin complexed with a set of such ligands and show that they interfere with several of the movements of tubulin subunits structural elements upon its transition from curved to straight. We also determined the structure of tubulin unliganded at the colchicine site; this reveals that a beta-tubulin loop (termed T7) flips into this site. As with colchicine site ligands, this prevents a helix which is at the interface with alpha-tubulin from stacking onto a beta-tubulin beta sheet as in straight protofilaments. Whereas in the presence of these ligands the interference with microtubule assembly gets frozen, by flipping in and out the beta-subunit T7 loop participates in a reversible way in the resistance to straightening that opposes microtubule assembly. Our results suggest that it thereby contributes to microtubule dynamic instability.
Conflict of interest statement
Conflict of interest statement: P.M. and V.M. are employees of the company that sells Taxotere, an anti-cancer drug that targets tubulin.
Figures
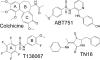

Similar articles
-
Structural Insights into the Inhibition of Tubulin by the Antitumor Agent 4β-(1,2,4-triazol-3-ylthio)-4-deoxypodophyllotoxin.ACS Chem Biol. 2017 Mar 17;12(3):746-752. doi: 10.1021/acschembio.6b00842. Epub 2017 Jan 25. ACS Chem Biol. 2017. PMID: 28035796
-
Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain.Nature. 2004 Mar 11;428(6979):198-202. doi: 10.1038/nature02393. Nature. 2004. PMID: 15014504
-
Stathmin and interfacial microtubule inhibitors recognize a naturally curved conformation of tubulin dimers.J Biol Chem. 2010 Oct 8;285(41):31672-81. doi: 10.1074/jbc.M110.141929. Epub 2010 Jul 30. J Biol Chem. 2010. PMID: 20675373 Free PMC article.
-
Recent advances in research of colchicine binding site inhibitors and their interaction modes with tubulin.Future Med Chem. 2021 May;13(9):839-858. doi: 10.4155/fmc-2020-0376. Epub 2021 Apr 6. Future Med Chem. 2021. PMID: 33821673 Review.
-
Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin.Med Res Rev. 2008 Jan;28(1):155-83. doi: 10.1002/med.20097. Med Res Rev. 2008. PMID: 17464966 Review.
Cited by
-
Mapping flexibility and the assembly switch of cell division protein FtsZ by computational and mutational approaches.J Biol Chem. 2010 Jul 16;285(29):22554-65. doi: 10.1074/jbc.M110.117127. Epub 2010 May 13. J Biol Chem. 2010. PMID: 20472561 Free PMC article.
-
Heterocyclic-Fused Pyrimidines as Novel Tubulin Polymerization Inhibitors Targeting the Colchicine Binding Site: Structural Basis and Antitumor Efficacy.J Med Chem. 2018 Feb 22;61(4):1704-1718. doi: 10.1021/acs.jmedchem.7b01858. Epub 2018 Feb 12. J Med Chem. 2018. PMID: 29406710 Free PMC article.
-
Identification of selective tubulin inhibitors as potential anti-trypanosomal agents.Bioorg Med Chem Lett. 2012 Sep 1;22(17):5508-16. doi: 10.1016/j.bmcl.2012.07.023. Epub 2012 Jul 14. Bioorg Med Chem Lett. 2012. PMID: 22850214 Free PMC article.
-
Developing novel C-4 analogues of pyrrole-based antitubulin agents: weak but critical hydrogen bonding in the colchicine site.Medchemcomm. 2013;4(2):417-421. doi: 10.1039/C2MD20320K. Medchemcomm. 2013. PMID: 23457660 Free PMC article.
-
(E)-4-aryl-4-oxo-2-butenoic acid amides, chalcone-aroylacrylic acid chimeras: design, antiproliferative activity and inhibition of tubulin polymerization.Eur J Med Chem. 2013 Apr;62:40-50. doi: 10.1016/j.ejmech.2013.01.006. Epub 2013 Jan 11. Eur J Med Chem. 2013. PMID: 23353745 Free PMC article.
References
-
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. - PubMed
-
- Vandecandelaere A, Brune M, Webb MR, Martin SR, Bayley PM. Phosphate release during microtubule assembly: What stabilizes growing microtubules? Biochemistry. 1999;38:8179–8188. - PubMed
-
- Lowe J, Li H, Downing KH, Nogales E. Refined structure of αβ-tubulin at 3.5 A resolution. J Mol Biol. 2001;313:1045–1057. - PubMed
-
- Ravelli RBG, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

