A synopsis of eukaryotic Nalpha-terminal acetyltransferases: nomenclature, subunits and substrates
- PMID: 19660095
- PMCID: PMC2722095
- DOI: 10.1186/1753-6561-3-S6-S2
A synopsis of eukaryotic Nalpha-terminal acetyltransferases: nomenclature, subunits and substrates
Abstract
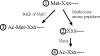
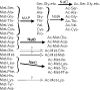
We have introduced a consistent nomenclature for the various subunits of the NatA-NatE N-terminal acetyltransferases from yeast, humans and other eukaryotes.
Figures
Similar articles
-
A novel human NatA Nalpha-terminal acetyltransferase complex: hNaa16p-hNaa10p (hNat2-hArd1).BMC Biochem. 2009 May 29;10:15. doi: 10.1186/1471-2091-10-15. BMC Biochem. 2009. PMID: 19480662 Free PMC article.
-
Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans.Proc Natl Acad Sci U S A. 2009 May 19;106(20):8157-62. doi: 10.1073/pnas.0901931106. Epub 2009 May 6. Proc Natl Acad Sci U S A. 2009. PMID: 19420222 Free PMC article.
-
N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins.J Mol Biol. 2003 Jan 24;325(4):595-622. doi: 10.1016/s0022-2836(02)01269-x. J Mol Biol. 2003. PMID: 12507466 Review.
-
Composition and biological significance of the human Nalpha-terminal acetyltransferases.BMC Proc. 2009 Aug 4;3 Suppl 6(Suppl 6):S3. doi: 10.1186/1753-6561-3-S6-S3. BMC Proc. 2009. PMID: 19660096 Free PMC article.
-
N-terminal acetylation and other functions of Nα-acetyltransferases.Biol Chem. 2012 Apr;393(4):291-8. doi: 10.1515/hsz-2011-0228. Biol Chem. 2012. PMID: 22718636 Review.
Cited by
-
The Not4 RING E3 Ligase: A Relevant Player in Cotranslational Quality Control.ISRN Mol Biol. 2013 Jan 21;2013:548359. doi: 10.1155/2013/548359. eCollection 2013. ISRN Mol Biol. 2013. PMID: 27335678 Free PMC article. Review.
-
Comparative large scale characterization of plant versus mammal proteins reveals similar and idiosyncratic N-α-acetylation features.Mol Cell Proteomics. 2012 Jun;11(6):M111.015131. doi: 10.1074/mcp.M111.015131. Epub 2012 Jan 5. Mol Cell Proteomics. 2012. PMID: 22223895 Free PMC article.
-
FIH permits NAA10 to catalyze the oxygen-dependent lysyl-acetylation of HIF-1α.Redox Biol. 2018 Oct;19:364-374. doi: 10.1016/j.redox.2018.09.002. Epub 2018 Sep 7. Redox Biol. 2018. PMID: 30237125 Free PMC article.
-
In silico identification and characterization of N-Terminal acetyltransferase genes of poplar (Populus trichocarpa).Int J Mol Sci. 2014 Jan 27;15(2):1852-64. doi: 10.3390/ijms15021852. Int J Mol Sci. 2014. PMID: 24473137 Free PMC article.
-
Using VAAST to identify an X-linked disorder resulting in lethality in male infants due to N-terminal acetyltransferase deficiency.Am J Hum Genet. 2011 Jul 15;89(1):28-43. doi: 10.1016/j.ajhg.2011.05.017. Epub 2011 Jun 23. Am J Hum Genet. 2011. PMID: 21700266 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

